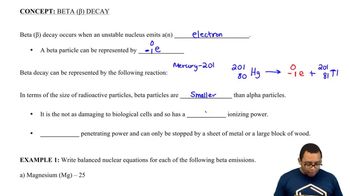
Identify each of the following as alpha decay, beta decay, positron emission, or gamma emission:
a. 12755Cs → 12754Xe + 0+1e
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 2:06m
2:06mMaster Types of Radiation Concept 1 with a bite sized video explanation from Jules
Start learning