Textbook Question
Complete the following equations:
b.
1359
views

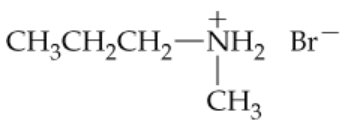
 Verified step by step guidance
Verified step by step guidance



Complete the following equations:
b.
Draw the structures corresponding to the following names:
a. N-Methylpentylamine
Name the following amines, and identify them as primary, secondary, or tertiary:
b.
Give names or structures for the following ammonium salts. Indicate whether each is the ammonium salt of a primary, secondary, or tertiary amine.
a.
Give names or structures for the following ammonium salts. Indicate whether each is the ammonium salt of a primary, secondary, or tertiary amine.
c. N-Butyl-N-isopropylhexylammonium chloride
Identify the functional groups in cocaine