Textbook Question
Which is the stronger base, trimethylamine or ammonia? In which direction will the following reaction proceed?
347
views

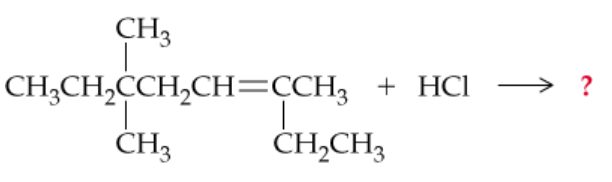
 Verified step by step guidance
Verified step by step guidance



Which is the stronger base, trimethylamine or ammonia? In which direction will the following reaction proceed?
How do amines differ from analogous alcohols in (a) odor, (b) basicity, and (c) boiling point?
Name the following compounds:
a.
Complete the following equations (Hint: Answers may include concepts learned from previous organic chapters):
c.
Complete the following equations (Hint: Answers may include concepts learned from previous organic chapters):
e.
Baeocystin is a hallucinogenic compound that is isolated from the mushroom Psilocybe baeocystis and has the structure shown below. What heterocyclic base (Table 16.1) is the parent of this compound?