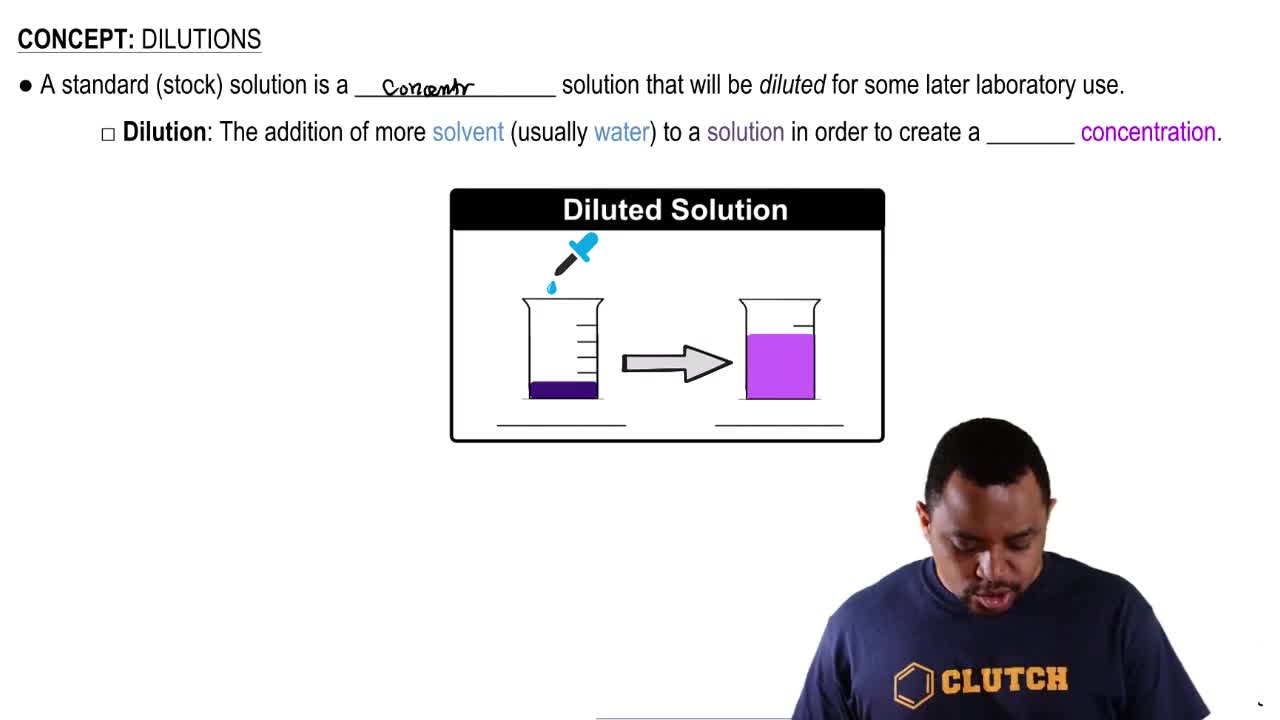
Calculate the mass/volume percent (m/v) for the solute in each of the following:
a. 75 g of Na2SO4 in 250 mL of Na2SO4 solution
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 0:52m
0:52mMaster Percent Concentrations Concept 1 with a bite sized video explanation from Jules
Start learning