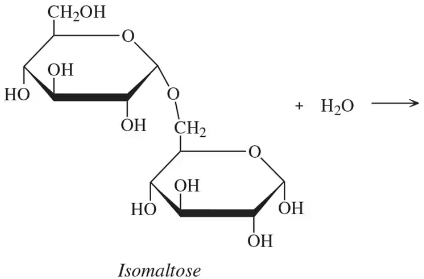
What are the disaccharides and polysaccharides present in each of the following?
a. <IMAGE>
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:39m
3:39mMaster Types of Disaccharides Concept 1 with a bite sized video explanation from Jules
Start learning