Indium consists of two isotopes, 11349In and 11549In. If the atomic mass for indium on the periodic table is 114.8, are there more atoms of 11349In or 11549In in a sample of indium?
Ch.4 Atoms and Elements

Timberlake13th EditionChemistry: An Introduction to General, Organic, and Biological ChemistryISBN: 9780134421353Not the one you use?Change textbook
Chapter 4, Problem 55e
Write the group number and draw the Lewis symbol for each of the following elements:
e. gallium
 Verified step by step guidance
Verified step by step guidance1
Determine the group number of gallium by locating it on the periodic table. Gallium is in Group 13 (or IIIA in older notation), which means it has 3 valence electrons.
Understand that the Lewis symbol represents the valence electrons of an atom as dots around the chemical symbol. Gallium has 3 valence electrons because it is in Group 13.
Write the chemical symbol for gallium, which is 'Ga'. This will serve as the central part of the Lewis symbol.
Place three dots around the 'Ga' symbol to represent the 3 valence electrons. Distribute the dots around the symbol, starting with one dot on each side before pairing them, if necessary.
Ensure the Lewis symbol is complete and correctly represents gallium's valence electrons. The final representation should show 'Ga' with three dots around it, indicating its valence electrons.

Verified video answer for a similar problem:
This video solution was recommended by our tutors as helpful for the problem above.
Video duration:
42sWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Lewis Symbols
Lewis symbols, also known as Lewis dot diagrams, represent the valence electrons of an atom as dots surrounding the element's symbol. This visual representation helps in understanding how atoms bond with each other, as it shows the number of electrons available for bonding. For example, the Lewis symbol for gallium (Ga) would depict its three valence electrons, which are crucial for its chemical behavior.
Recommended video:
Guided course
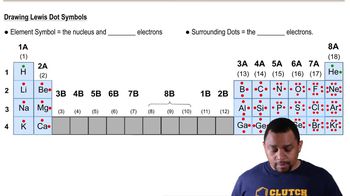
Lewis Dot Symbols (Simplified) Concept 2
Valence Electrons
Valence electrons are the electrons in the outermost shell of an atom and are key to determining how an element interacts chemically with others. The number of valence electrons influences an element's reactivity, bonding capacity, and overall chemical properties. Gallium, with its three valence electrons, typically forms bonds by sharing or losing these electrons in chemical reactions.
Recommended video:
Guided course
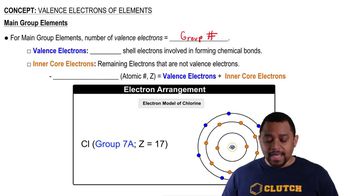
Valence Electrons of Elements (Simplified) Concept 1
Group Number
The group number in the periodic table indicates the column in which an element is found and reflects the number of valence electrons in its atoms. Elements in the same group often exhibit similar chemical properties due to their comparable electron configurations. Gallium is located in Group 13, which signifies that it has three valence electrons and shares characteristics with other elements in that group, such as aluminum.
Recommended video:
Guided course

Calculate Oxidation Numbers
Related Practice
Textbook Question
1439
views
Textbook Question
What is the group number and number of valence electrons for each of the following elements?
e. barium
1680
views
Textbook Question
Write the group number and draw the Lewis symbol for each of the following elements:
c. calcium
1301
views
Textbook Question
Fill in the following blanks using larger or smaller, higher or lower. Mg has a _______ atomic size and a _______ ionization energy than Cs.
1378
views
Textbook Question
Complete each of the statements a to d using 1, 2, or 3:
1. decreases
2. increases
3. remains the same
Going down Group 6A (16),
d. the number of valence electrons _________
1250
views
Textbook Question
Complete each of the statements a to d using 1, 2, or 3:
1. decreases
2. increases
3. remains the same
Going from left to right across Period 4,
d. the number of valence electrons ________
1975
views
