Isoleucine has the zwitterion structure shown. Draw the structure and give the net charge of isoleucine that will predominate at the indicated pH values (pI = 6.0).
b. pH 6.0
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:
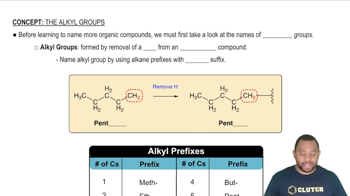
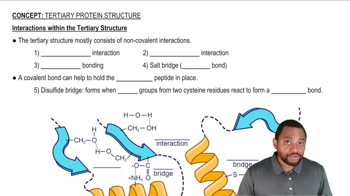

 5:16m
5:16mMaster Nonpolar Amino Acids Concept 1 with a bite sized video explanation from Jules
Start learning