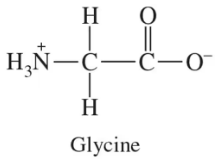
Give the three-letter and one-letter abbreviations and identify the functional group in the side chain for each amino acid in Problem 10.1.
a. alanine
b. lysine
c. tryptophan
d. aspartate
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 5:16m
5:16mMaster Nonpolar Amino Acids Concept 1 with a bite sized video explanation from Jules
Start learning