What alkene could you use to make the following products? Draw the structure of the alkene, and tell what other reagent is also required for the reaction to occur.
d.
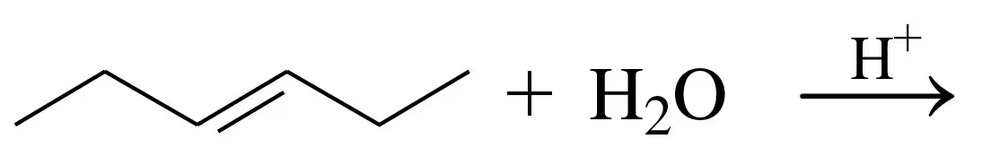
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 1:25m
1:25mMaster Symmetric Alkene Hydration Concept 1 with a bite sized video explanation from Jules
Start learning