Suppose you have a sample of benzoic acid dissolved in water.
b. Now assume that aqueous NaOH is added to the benzoic acid solution until pH 12 is reached. Draw the structure of the major organic species present.
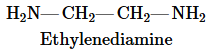
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: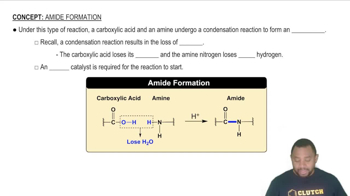



 1:9m
1:9mMaster Acid-Base Reactions Concept 1 with a bite sized video explanation from Jules
Start learning