Using condensed structural formulas, or line-angle formulas if cyclic, write the balanced chemical equations for the (1) reaction of each of the following amines with water and (2) neutralization with HCl:
c. aniline

 Verified step by step guidance
Verified step by step guidance

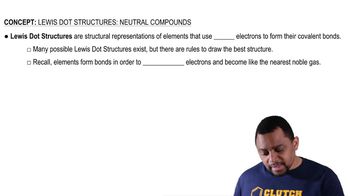
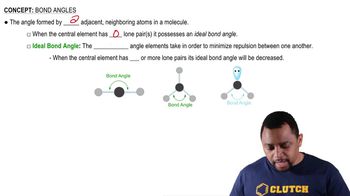
Using condensed structural formulas, or line-angle formulas if cyclic, write the balanced chemical equations for the (1) reaction of each of the following amines with water and (2) neutralization with HCl:
c. aniline
Using condensed structural formulas, or line-angle formulas if cyclic, write the balanced chemical equations for the (1) reaction of each of the following amines with water and (2) neutralization with HBr:
b. propylamine
Using condensed structural formulas, or line-angle formulas if cyclic, write the balanced chemical equations for the (1) reaction of each of the following amines with water and (2) neutralization with HBr:
c. N-methylaniline
Draw the condensed structural or line-angle formula for the amide formed in each of the following reactions:
a.
Draw the condensed structural or line-angle formula for the amide formed in each of the following reactions:
c.
Draw the condensed structural or line-angle formula for the amide formed in each of the following reactions:
c.