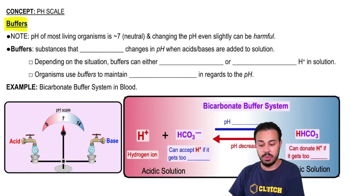
In a protein buffer system, if the pH increases:
(a) The protein acquires a hydrogen ion from carbonic acid
(b) Hydrogen ions are buffered by hemoglobin molecules
(c) A hydrogen ion is released and a carboxylate ion is formed
(d) A chloride shift occurs