2. Chemistry
Introduction to Chemical Bonding
2. Chemistry
Introduction to Chemical Bonding
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Open Question
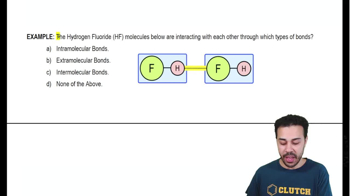
Appropriately label all of the chemical bonds in this image as either intramolecular or intermolecular.
2286views141rank - Multiple Choice
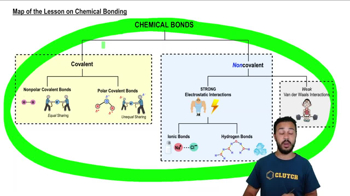
Map of the Lesson on Chemical Bonding
According to the map above, which of the following are types of covalent bonds?
5856views72rank - Multiple ChoiceHow would you respond to this reasoning? Oxygen is not a greenhouse gas; therefore, gases containing oxygen—such as ozone, nitrous oxide, and carbon dioxide—are not greenhouse gases either.4192views
- Multiple ChoiceAn atom that normally has __________ in its outer shell would not tend to form chemical bonds with other atoms.1639views1rank
- Multiple ChoiceThe chemical characteristics or reactivity of an element depend mostly on the __________.2266views