14. Solutions
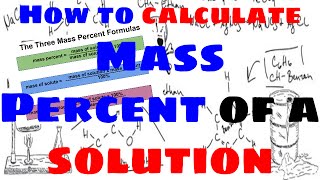
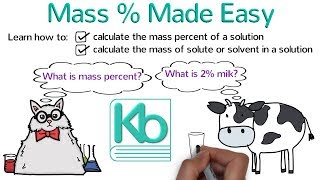
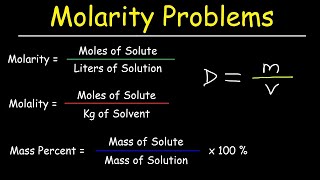
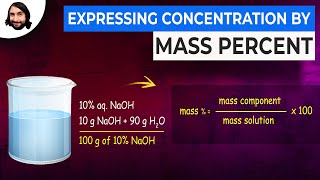
Solutions: Mass Percent
14. Solutions
Solutions: Mass Percent
Additional 3 creators.
Learn with other creators
Showing 6 of 6 videos
Practice this topic
- Multiple Choice
Calculate the amount of water (in kilograms) that must be added to 12.0 g of urea, (NH2)2CO, in the preparation of a 18.3 percent by mass solution. The molar mass of urea, (NH2)2CO, is 60.055 g/mol.
1064views3rank - Multiple Choice
A solution was prepared by dissolving 51.0 g of KBr in 310 mL of water. Calculate the mass percent of KBr in the solution.
989views4rank - Multiple Choice
An aqueous LiNO2 solution is made using 90.3 g LiNO2 and diluting it to a total volume of 1.72 L. If the density of the solution is 1.20 g/mL, what is the mass percent of the solution?
1001views - Multiple Choice
Determine the percent sulfuric acid by mass of a 1.37 m aqueous solution of H2SO4.
971views4rank