Classify each of the following monosaccharides as an aldopentose, ketopentose, aldohexose, or ketohexose:
a. Psicose is present in low amounts in foods.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: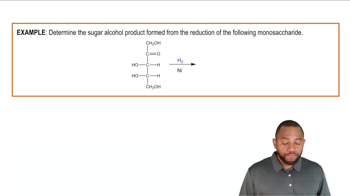


 1:48m
1:48mMaster Intro to Carbohydrates Concept 1 with a bite sized video explanation from Jules
Start learning