Avobenzone is a common ingredient in sunscreen. Its structural formula is shown.
b. What is the molecular formula and molar mass of avobenzone?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: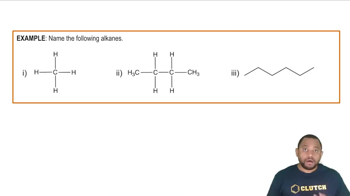


 2:26m
2:26mMaster Skeletal Formula Concept 1 with a bite sized video explanation from Jules
Start learning