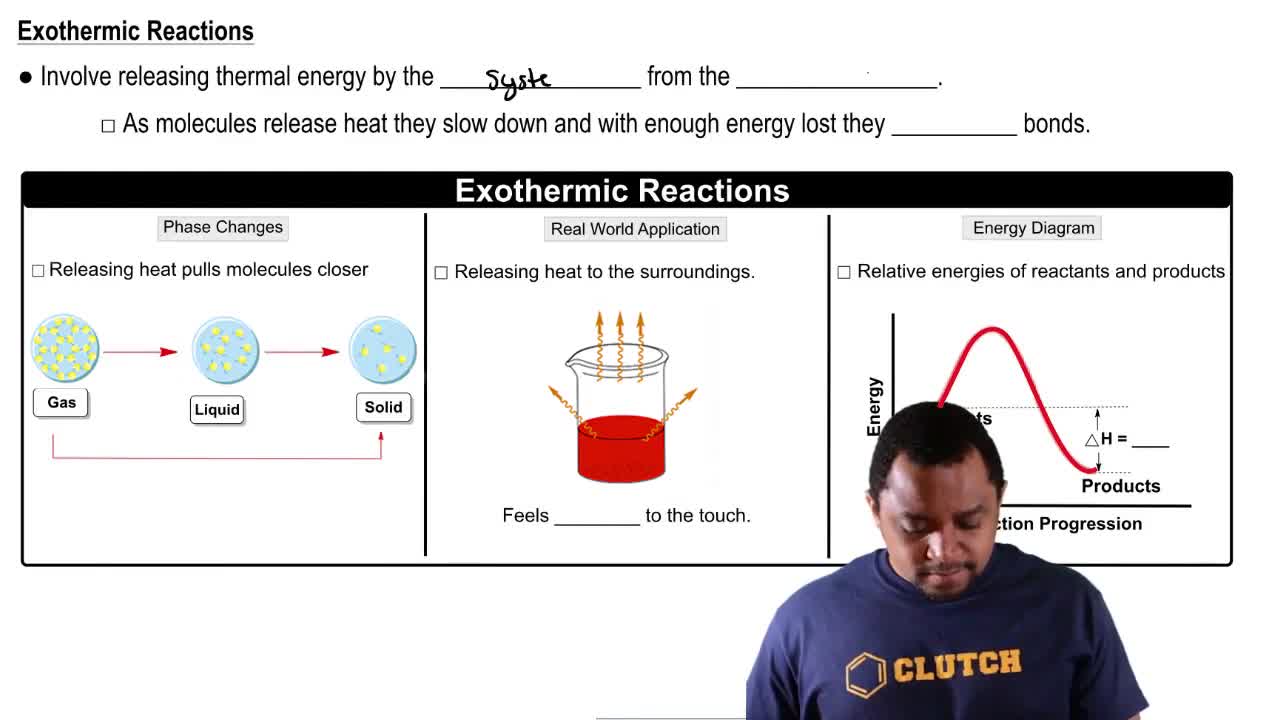
Classify each of the following as exothermic or endothermic:
c. The metabolism of glucose in the body provides energy.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 2:30m
2:30mMaster Endothermic & Exothermic Reactions with a bite sized video explanation from Jules
Start learning