Textbook Question
Name the types of chemical reactions that tend to be found in anabolic pathways.
719
views

 Verified step by step guidance
Verified step by step guidance


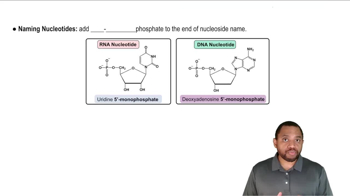
Name the types of chemical reactions that tend to be found in anabolic pathways.
Identify the metabolic nucleotide described by the following:
a. contains the vitamin riboflavin
Identify the metabolic nucleotide described by the following:
a. contains a form of the vitamin niacin
Using abbreviations (not structures), write the reaction of flavin adenine dinucleotide that gives off energy (–∆G) .
Name a carbohydrate (if any) that undergoes digestion in each of the following sites:
a. mouth
Name a carbohydrate (if any) that undergoes digestion in each of the following sites:
c. small intestine