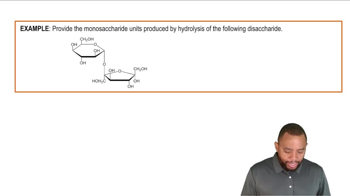
Which of the following is a cofactor and which is a coenzyme?
b. Pyridoxyl phosphate
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 2:23m
2:23mMaster Intro to Coenzymes Concept 1 with a bite sized video explanation from Jules
Start learning