Textbook Question
How many molecules of acetyl-CoA result from catabolism of 1 molecule of glyceryl trilaurate?
499
views

 Verified step by step guidance
Verified step by step guidance
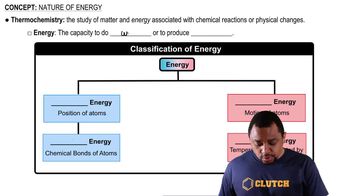


How many molecules of acetyl-CoA result from catabolism of 1 molecule of glyceryl trilaurate?
Why is the stepwise oxidation of fatty acids called β oxidation?
How many moles of ATP are produced by the complete oxidation of 1 mol of myristic acid?
Show the products of each step in the fatty acid oxidation of hexanoic acid.
a.
Write the equation for the final step in the catabolism of any fatty acid with an even number of carbons.
How many molecules of acetyl-CoA result from complete catabolism of the following compounds?
a. Myristic acid, CH3(CH2)12COOH