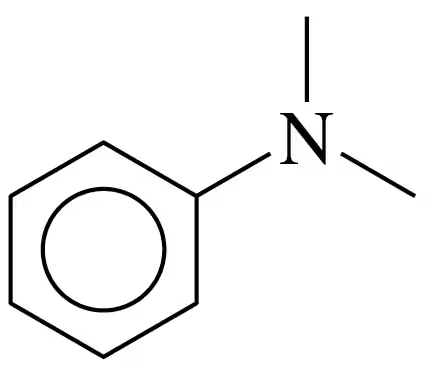
Draw the condensed structural formula, or line-angle formula if cyclic, for each of the following amines:
c. butylpropylamine
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 :28m
:28mMaster Common Naming: Amines Concept 1 with a bite sized video explanation from Jules
Start learning