In the presence of a small amount of bromine, the following light-promoted reaction has been observed.
b. Explain why only this one type of hydrogen atom has been replaced, in preference to any of the other hydrogen atoms in the starting material.
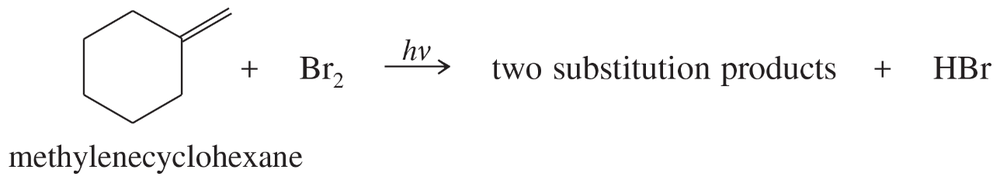
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 6:15m
6:15mMaster The general mechanism of Allylic Halogenation. with a bite sized video explanation from Johnny
Start learning