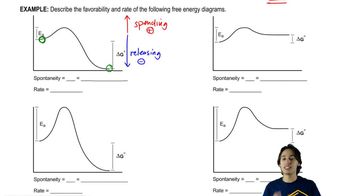
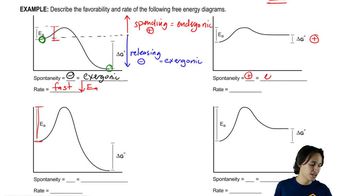
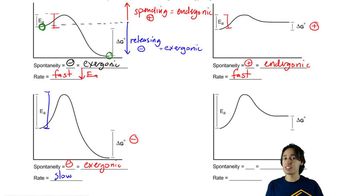
6. Thermodynamics and Kinetics
Energy Diagram
6. Thermodynamics and Kinetics
Energy Diagram
Showing 5 of 5 videos
Practice this topic
- Multiple ChoiceIf the transition state has a high energy relative to the energies of the reactants and products, what does this say about the reaction?868views
- Multiple ChoiceWhich equation would you use to calculate the rate of a reaction?947views
- Multiple ChoiceComplete the following sentence: Breaking bonds ____________.926views
- Multiple ChoiceWhich reaction coordinate diagram matches the following acid/base reaction?845views
- Textbook Question
The bond angles in a regular polygon with n sides are equal to 180° - 360°/n
a. What are the bond angles in a regular octagon?
b. What are the bond angles in a regular nonagon?
1367views - Textbook QuestionThe bromination of methane proceeds through the following steps:1. Br2 + 2 Br• ΔH° (per mole)/+190 kJ (45 kcal)Ea (per mole)/ 190 kJ (45 kcal)2. CH4 + Br• —> CH3+ HBr +73 kJ (17 kcal) 79 kJ (19 kcal) 3. • CH3 + Br2 —> CH3Br + Br -112 kJ (-27 kcal) 4 kJ (1 kcal) a. Draw a complete reaction-energy diagram for this reaction. b. Label the rate-limiting step.3345views
- Textbook Question
Draw a reaction-energy diagram for a one-step exothermic reaction. Label the parts that represent the reactants, products, transition state, activation energy, and heat of reaction.
2037views - Textbook Question
The bromination of methane proceeds through the following steps:
a. Draw a complete reaction-energy diagram for this reaction.
b. Label the rate-limiting step.
1303views