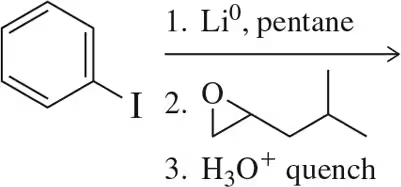Back
BackProblem 2b
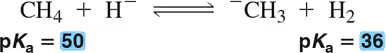
Using pKₐ values, calculate the equilibrium constants for the following acid–base reactions.
(b)
Problem 3
Suggest a reagent for the transformation of a 1° alcohol to a 1° alkyl halide.
Problem 4b
Give the oxidation state of the palladium in each of the following forms.
(b) AcO―Pd―OAc
Problem 4c
Give the oxidation state of the palladium in each of the following forms.
(c) Pd(PPh₃)₄
Problem 4d
Give the oxidation state of the palladium in each of the following forms.
(d) H₃C―Pd―Br
Problem 5b
Calculate Keq for the following acid–base reactions.
(b)
Problem 6a
Predict the product that would form when a Grignard reagent is prepared in the presence of deuterated water.
Problem 7
Predict the product that would result from the reaction of an organolithium reagent with a ketone when a hydroxyl group is present in the ketone substrate.
Problem 8a
Predict the product of the following epoxide addition reactions.
(a)
Problem 8b
Predict the product of the following epoxide addition reactions.
(b)
Problem 9
Addition to an epoxide occurs via an SN2 reaction, but the stereochemistry of the epoxide is retained in the following reaction. Why?
Problem 10
Working backward, design a synthesis of the following alcohol using two different epoxide/Grignard reagent combinations.
Problem 11
Using the epoxide shown, addition of an organolithium reagent, when followed by an acid quench, produces only the starting epoxy alcohol. Why? How could the reaction be modified to produce the desired molecule? [Hint: Look back at Section 13.14.]
Problem 12a
Predict the product of the following aldehyde and ketone addition reactions.
(a)
Problem 12b
Predict the product of the following aldehyde and ketone addition reactions.
(b)
Problem 12c
Predict the product of the following aldehyde and ketone addition reactions.
(c)
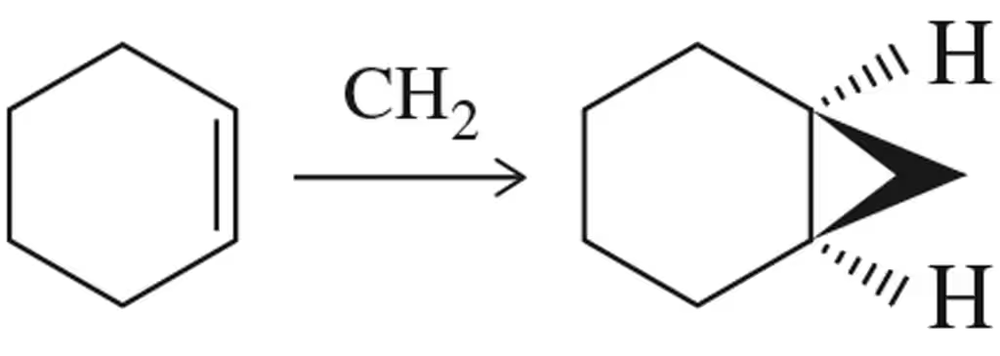
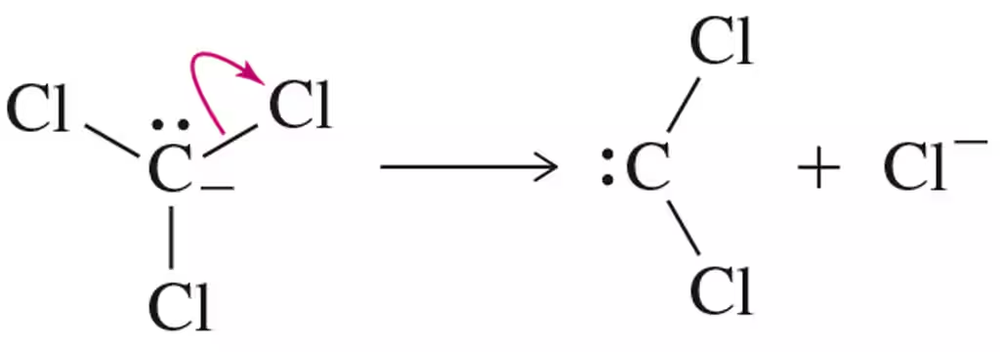
Problem 16
Provide an arrow-pushing mechanism for the cyclopropanation of cyclohexene with methylene carbene. Rationalize the outcome.
Problem 17
Estimate the value of entropy (∆S > 0 or ∆S < 0) for the elimination step shown.