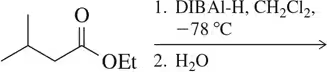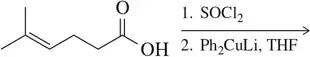Back
Back Mullins 1st Edition
Mullins 1st Edition Ch. 19 - Nucleophilic Acyl Substitution II: Carboxylic Acid Derivatives
Ch. 19 - Nucleophilic Acyl Substitution II: Carboxylic Acid DerivativesProblem 1
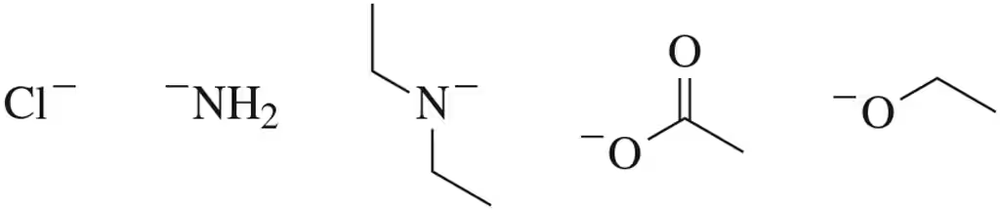
Rank the following anions based on their stability as potential leaving groups. Explain your reasoning (1 = most stable ; 5 = least stable).
Problem 2
Would you expect a ketone or an ester to be more reactive with a strong nucleophile? Justify your answer.
Problem 7a
Which of the following are considered carboxylic acid derivatives?
(a)
Problem 9d
Provide the IUPAC name for the following molecules.
(d)
Problem 9e
Provide the IUPAC name for the following molecules.
(e)
Problem 10a
Provide a molecular structure that corresponds to the given name.
(a) benzoic propanoic anhydride
Problem 10e
Provide a molecular structure that corresponds to the given name.
(e) (R)-N,N-diethyl 5-cyclohexyl-3-methoxypentanamide
Problem 11b
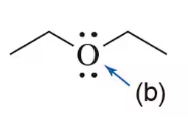
Predict the hybridization of the indicated atoms.
(b)
Problem 14
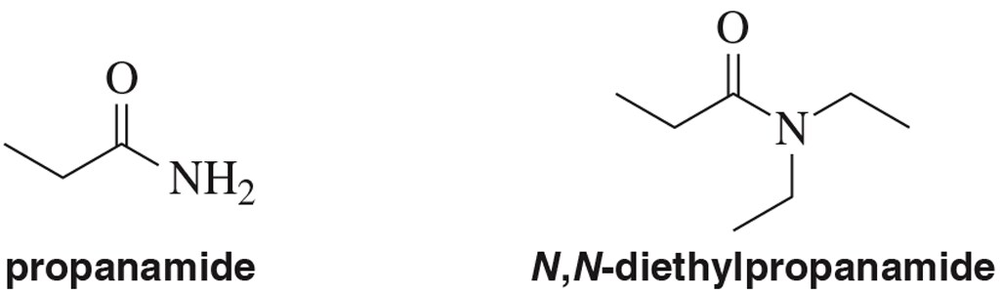
How would you expect the IR and ¹H NMR spectra for propanamide and N,N-diethylpropanamide to differ?
Problem 15
You might expect that aldehydes and ketones could undergo the addition/elimination mechanism. With strong nucleophiles, however, nucleophilic addition is the only outcome. Why?
Problem 24
A chemist unsuccessfully attempted to produce the 1,4-cyclohexanediol by hydration of the cyclohexene shown.
(a) Provide a mechanism for the formation of the actual product.
(b) Suggest a pathway using acetyl chloride as a protecting group that will allow for the formation of the desired product.
Problem 36a
Predict the product of the following reductions.
(a)
Problem 36b
Predict the product of the following reductions.
(b)
Problem 36c
Predict the product of the following reductions.
(c)
Problem 36d
Predict the product of the following reductions.
(d)
Problem 37
Show a mechanism for the lithium aluminum hydride reduction of benzoic anhydride.
Problem 38
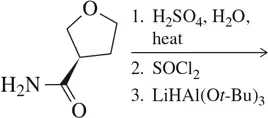
Predict the product of the multistep synthesis reaction shown.
Problem 39a
Predict the product of the following reaction sequences.
(a)
Problem 39b
Predict the product of the following reaction sequences.
(b)
Problem 39c
Predict the product of the following reaction sequences.
(c)
Problem 40a
The esters shown differ only by the alkoxy group.
(i) Predict the product(s) obtained when these react with DIBAl-H.
(ii) Based on your answer, in a sequence like this, would there ever be a need to convert from one ester to another?
(a)
Problem 40b
The esters shown differ only by the alkoxy group.
(i) Predict the product(s) obtained when these react with DIBAl-H.
(ii) Based on your answer, in a sequence like this, would there ever be a need to convert from one ester to another?
(b)
Problem 41
When the ester attacked the aluminum of DIBAl-H, why did the carbonyl oxygen attack preferentially over the alkoxy oxygen?
Problem 46
Why is a ketone more reactive/electrophilic than an ester?
Problem 47a
Predict the product of the following reactions.
(a)
Problem 47b
Predict the product of the following reactions.
(b)
Problem 47c
Predict the product of the following reactions.
(c)
Problem 48a
Suggest a series of steps involving a cuprate reagent that would convert the reactant on the left to the product on the right. The ideal number of steps is shown.
(a)
Problem 48b
Suggest a series of steps involving a cuprate reagent that would convert the reactant on the left to the product on the right. The ideal number of steps is shown.
(b)
Problem 48c
Suggest a series of steps involving a cuprate reagent that would convert the reactant on the left to the product on the right. The ideal number of steps is shown.
(c)