The site of production of cholecystokinin is:
a. The stomach
b. The small intestine
c. The pancreas
d. The large intestine
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: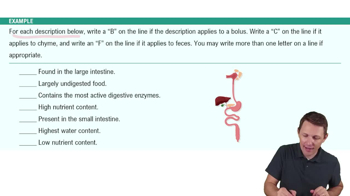

 6:54m
6:54mMaster Gallbladder and Bile with a bite sized video explanation from Bruce Bryan
Start learning