Sucrose and D-glucose rotate plane-polarized light to the right; D-fructose rotates light to the left. When sucrose is hydrolyzed, the glucose–fructose mixture rotates light to the left.
b. Why do you think the mixture is called “invert sugar”?
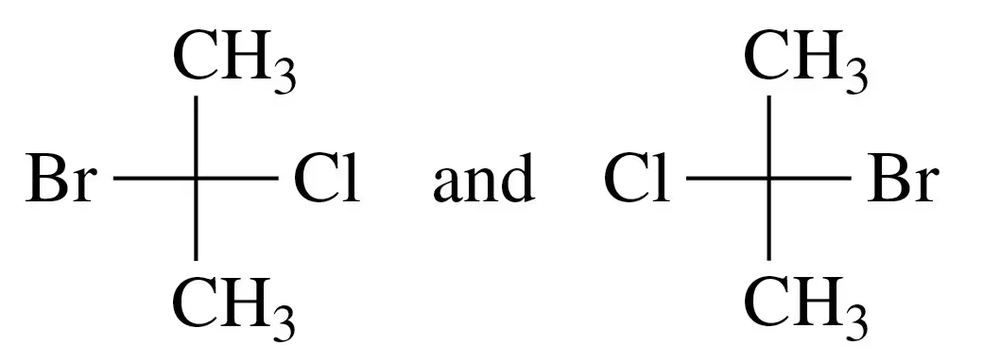
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:33m
3:33mMaster Enantiomers vs Diastereomers Concept 1 with a bite sized video explanation from Jules
Start learning