Identify each of the following pairs of Fischer projections as enantiomers or identical compounds:
a.
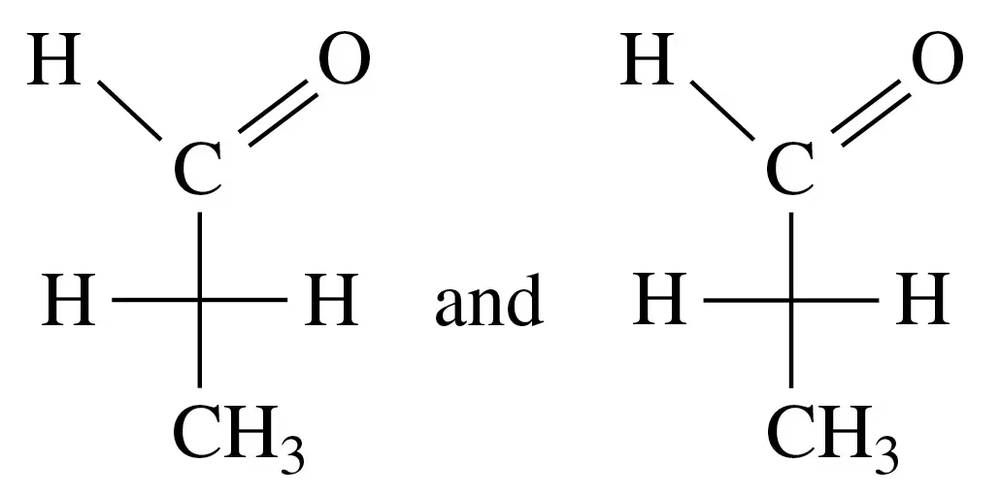
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:



 3:33m
3:33mMaster Enantiomers vs Diastereomers Concept 1 with a bite sized video explanation from Jules
Start learning