Textbook Question
Draw a structure for a compound that meets each of the following descriptions:
c. An alpha-bromoaldehyde, C4H7BrO
839
views

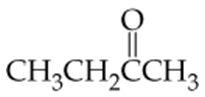
 Verified step by step guidance
Verified step by step guidance
Draw a structure for a compound that meets each of the following descriptions:
c. An alpha-bromoaldehyde, C4H7BrO
Draw a structure for a compound that meets each of the following descriptions:
b. An 8-carbon ketone with six carbons as its longest chain
Draw a structure for a compound that meets each of the following descriptions:
d. A cyclic alpha-hydroxyketone, C5H8O2
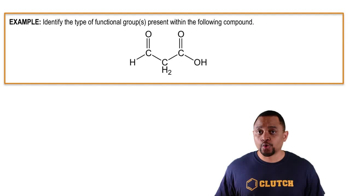
Indicate which compounds contain aldehyde or ketone carbonyl groups.
c. CH3CH2–O–CH2–CHO
Indicate which compounds contain aldehyde or ketone carbonyl groups.
f.
Redraw each of the following in line structure format. Indicate which compounds have an aldehyde carbonyl group, a ketone carbonyl group, or neither.
c.