Textbook Question
Draw the condensed structural formula for each of the following:
b. 4-chloro-2-pentanone
858
views

 Timberlake 13th Edition
Timberlake 13th Edition Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones
Ch.12 Alcohols, Thiols, Ethers, Aldehydes, and Ketones Problem 22b
Problem 22b Verified step by step guidance
Verified step by step guidance

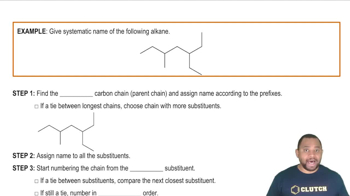

Draw the condensed structural formula for each of the following:
b. 4-chloro-2-pentanone
Draw the condensed structural formula for each of the following:
c. butyl methyl ketone
Draw the condensed structural formula for each of the following:
d. 3-methylpentanal
Which compound in each of the following pairs would be more soluble in water? Explain.
b. propanone or 3-hexanone
Which compound in each of the following pairs would be more soluble in water? Explain.
c. butanal or hexanal
Write the balanced chemical equation for the complete combustion of each of the following compounds:
b. 3-hexanol