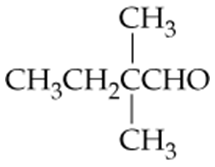
Give systematic, IUPAC names for the following compounds. Redraw each in line structure format.
a.
b.
c.
d. Dipropyl ketone
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:


 :54m
:54mMaster IUPAC Rules for Naming Aldehydes Concept 1 with a bite sized video explanation from Jules
Start learning