What ketones or aldehydes might be reduced to yield the following alcohols?
a.
b.
c. HOCH2–CH2–CH2OH
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: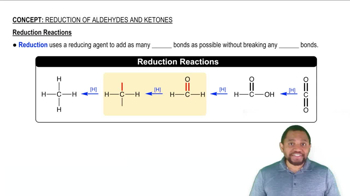
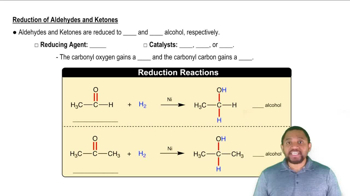


 2:38m
2:38mMaster Reduction Reactions Concept 1 with a bite sized video explanation from Jules
Start learning