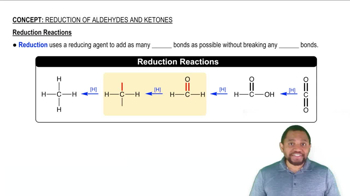
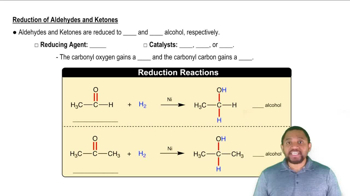
The carbonyl group can be reduced by addition of a hydride ion (H–) and (H+) a proton. Removal of H– and H+ from an alcohol results in a carbonyl group.
b. In the reaction, indicate which direction represents reduction and which represents oxidation.