Draw the condensed structural formula for the alcohol formed when each of the following is reduced by hydrogen in the presence of a nickel catalyst:
d. 2-methyl-3-pentanone
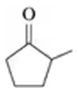
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: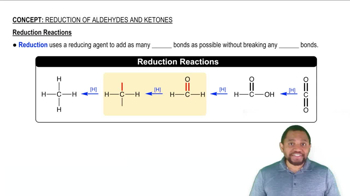
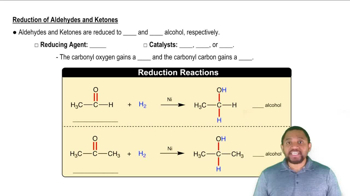


 2:38m
2:38mMaster Reduction Reactions Concept 1 with a bite sized video explanation from Jules
Start learning