Identify the following as containing soluble or insoluble fiber:
(a) oatmeal

 Verified step by step guidance
Verified step by step guidance


Identify the following as containing soluble or insoluble fiber:
(a) oatmeal
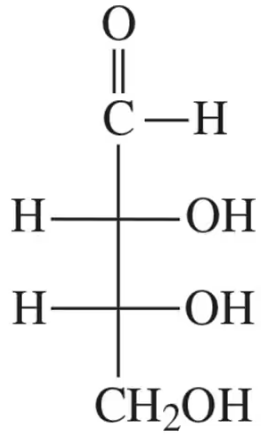
Classify each of the following monosaccharides by the type of carbonyl group and the number of carbons (for example, a monosaccharide with an aldehyde and three carbons is an aldotriose).
(a)
Classify each of the following monosaccharides by the type of carbonyl group and the number of carbons (for example, a monosaccharide with an aldehyde and three carbons is an aldotriose).
(a)
Draw the Fischer projection for the enantiomer (mirror image) of each of the following:
(a)
Classify structures A, B, and C in the figure as being either an enantiomer or a diastereomer of D-galactose.
Use the structure of D-galactose in Problem 6.15 to answer the following:
(a) Draw the Fischer projection of the carbon 3 epimer.