Textbook Question
Determine whether each of the following organic reactions is an oxidation or a reduction reaction. (Only the organic compounds are shown.)
(b)
587
views

 Verified step by step guidance
Verified step by step guidance


Determine whether each of the following organic reactions is an oxidation or a reduction reaction. (Only the organic compounds are shown.)
(b)
Write the products of the following reactions:
(b)
Write the products of the following reactions:
(a)
Epinephrine is the active ingredient in the EpiPen® used to treat severe allergic reactions. EpiPens expire due to the oxidation of the epinephrine. One of these reactions is shown below. Circle the groups in the product that were oxidized.
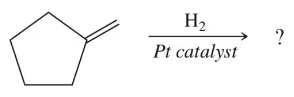
Fill in the missing organic products for the complete hydrogenation of the following:
(a)
Fill in the missing organic products for the complete hydrogenation of the following:
(c)