Textbook Question
Are the following compounds structural isomers, cis–trans isomers, or enantiomers?
(c)
779
views

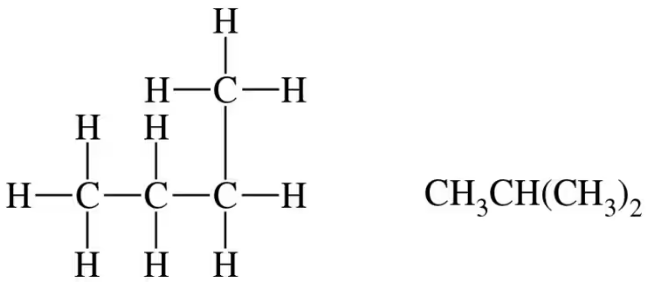
 Verified step by step guidance
Verified step by step guidance



Are the following compounds structural isomers, cis–trans isomers, or enantiomers?
(c)
Draw the enantiomer of each of the following compounds. If the compound is not chiral, state that fact.
(c)
Certain omega-3 fatty acids can be found only in animal sources, such as fatty fish. Two of these are eicosapentaenoic acid (EPA) [20:5] and docosahexaenoic acid (DHA) [22:6], both of which are ω-3 fatty acids. DHA has been shown to be important in healthy brain development, so it has recently been added to infant formulas. Breast milk is rich in DHA as long as the mother maintains a healthy diet that includes fish. Draw skeletal structures of the fatty acids EPA and DHA.