In photosynthesis, green plants convert carbon dioxide and water into glucose (C6H12O6) according to the following equation:
6 CO2(g) + 6 H2O(l) → C6H12O6(aq) + 6 O2(g)
b. Is the reaction endothermic or exothermic?
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: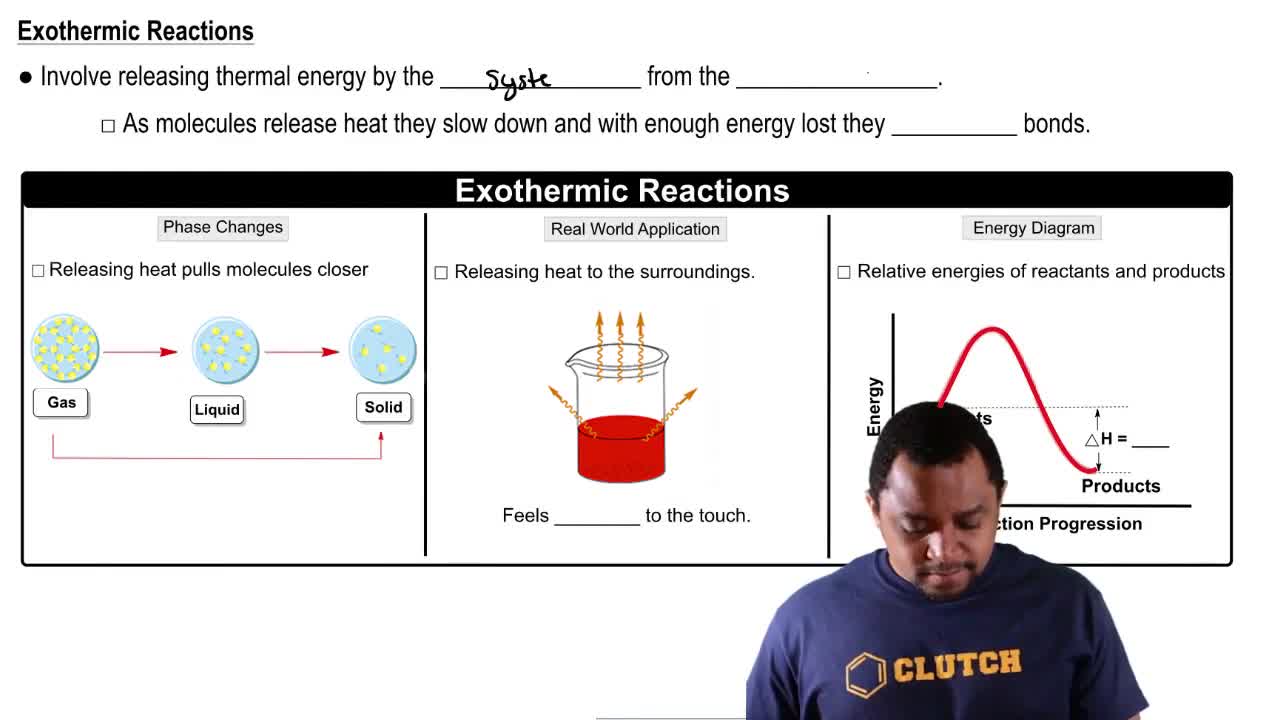



 2:30m
2:30mMaster Endothermic & Exothermic Reactions with a bite sized video explanation from Jules
Start learning