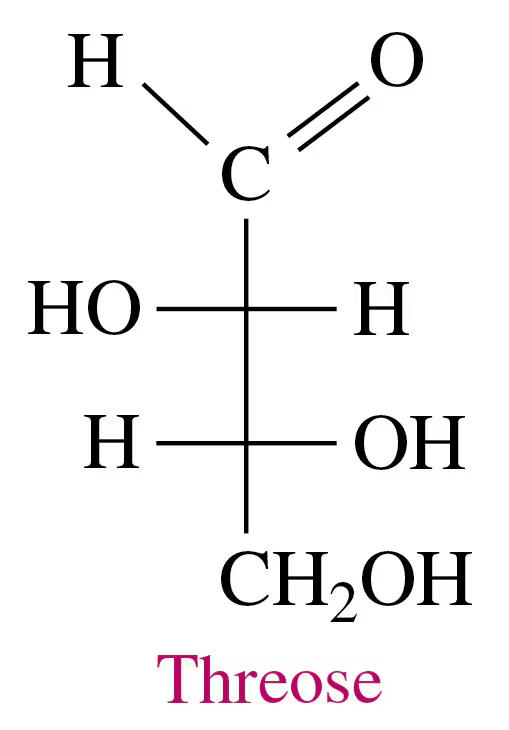
Textbook Question
Indicate whether each pair of Fischer projections represents enantiomers or identical structures.
a.
581
views

 Verified step by step guidance
Verified step by step guidance



Indicate whether each pair of Fischer projections represents enantiomers or identical structures.
a.
Indicate whether each pair of Fischer projections represents enantiomers or identical structures.
b.
Identify each of the following as D or L:
a.
Draw the Fischer projection for the other enantiomer of a to b in problem 13.21.
a.
b.
Draw the Fischer projection for the other enantiomer of c to d in problem 13.21.
c.
d.
Draw the Fischer projections for D-glucose and L-glucose.