Estimate the equilibrium composition of the chair conformers of the following cyclohexanes at room temp:
cis-1,3-diethylcyclohexane
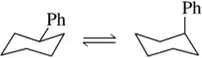
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: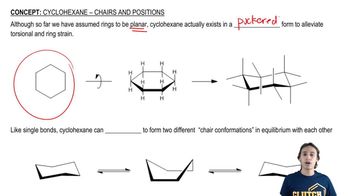



 6:27m
6:27mMaster Calculating Chair Equilibrium with a bite sized video explanation from Johnny
Start learning