SN1 substitution and E1 elimination frequently compete in the same reaction.
a. Propose a mechanism and predict the products for the solvolysis of 2-bromo-2,3,3-trimethylbutane in methanol.
 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem: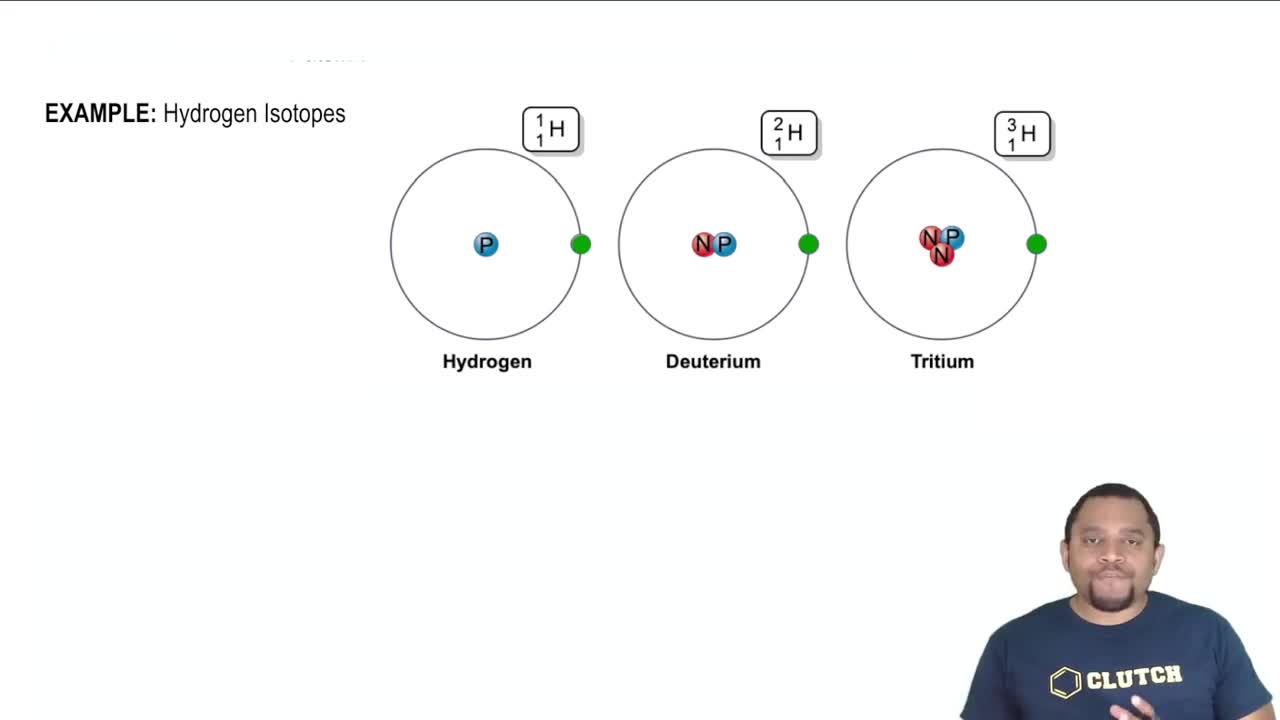


 0:38m
0:38mMaster Intro to Substitution/Elimination Problems with a bite sized video explanation from Johnny
Start learning