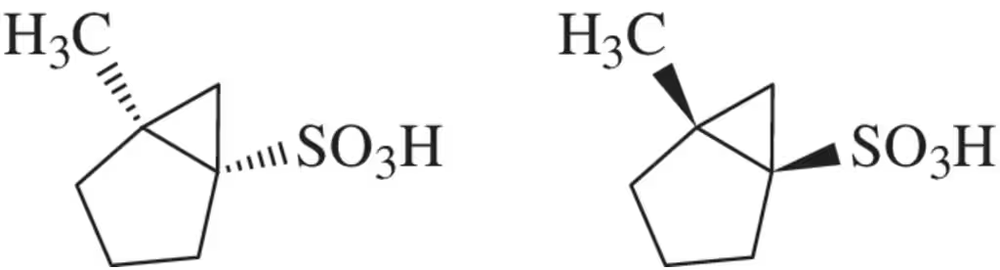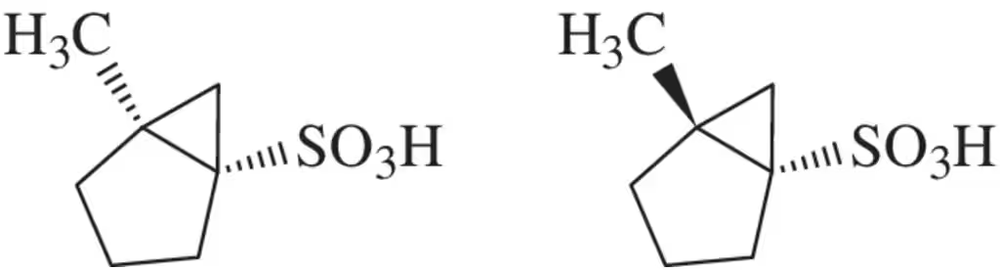Back
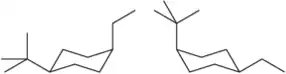
BackProblem 47d
Define the relationship between each set of two molecules as chain isomers, positional isomers, functional group isomers, enantiomers, diastereomers, conformational isomers, or identical
(d)
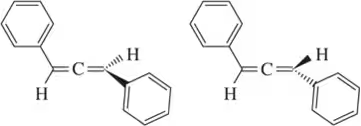
Problem 47e
Define the relationship between each set of two molecules as chain isomers, positional isomers, functional group isomers, enantiomers, diastereomers, conformational isomers, or identical
(e)
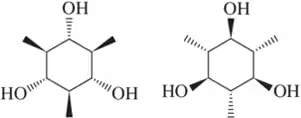
Problem 47f
Define the relationship between each set of two molecules as chain isomers, positional isomers, functional group isomers, enantiomers, diastereomers, conformational isomers, or identical
(f)
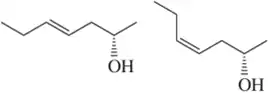
Problem 47h
Define the relationship between each set of two molecules as chain isomers, positional isomers, functional group isomers, enantiomers, diastereomers, conformational isomers, or identical
(h)
Problem 50a
Assign the absolute stereochemistry for each of the following molecules drawn in their Fischer projection.
(a)
Problem 50d
Assign the absolute stereochemistry for each of the following molecules drawn in their Fischer projection.
(d)
Problem 51e
Assign the absolute stereochemistry for each of the following molecules.
(e)
Problem 51f
Assign the absolute stereochemistry for each of the following molecules.
(f)
Problem 52a
Complete the structure of each of these so that it matches the (R) or (S) configuration associated with the name.
(a)
Problem 52d
Complete the structure of each of these so that it matches the (R) or (S) configuration associated with the name.
(d)
Problem 52e
Complete the structure of each of these so that it matches the (R) or (S) configuration associated with the name.
(e)
Problem 53
Draw the enantiomer of each of the molecules you drew in Assessment 6.52.
Problem 54
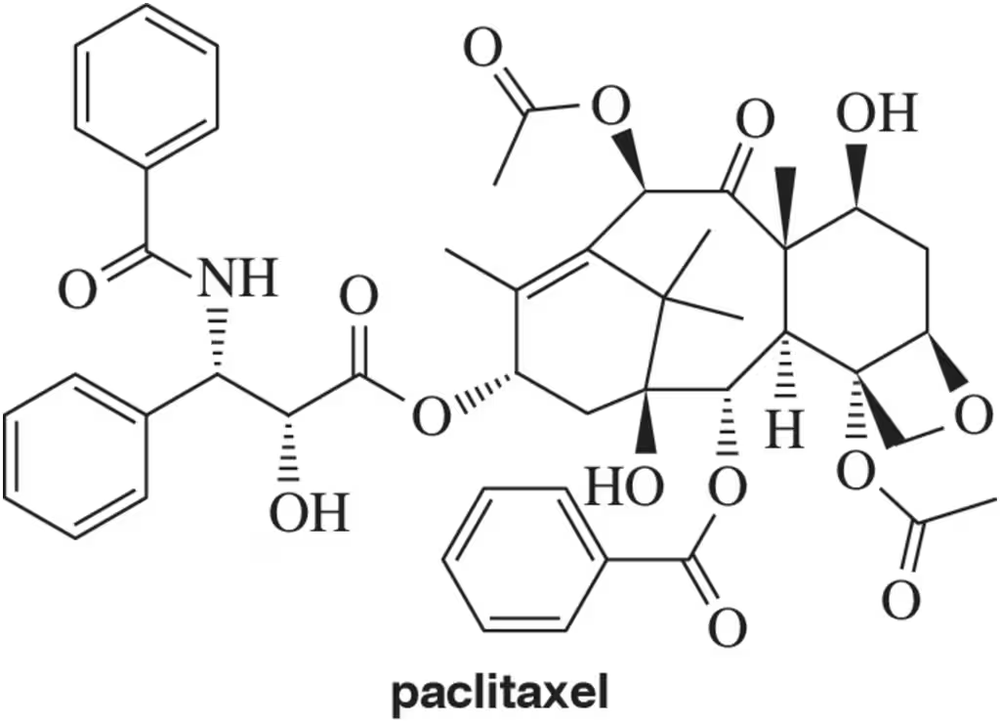
Natural products are organic compounds produced by living organisms, including plants, fungi, and animals. Often referred to as secondary metabolites because they are not required for survival of the organism, natural products have found broad utility as drugs themselves or as lead compounds used in the development of medicines. One such example is paclitaxel, which has been used as a cancer drug. Paclitaxel is isolated from the yew tree, where it is produced as a single stereoisomer (shown). Based on its structure, how many stereoisomers are possible for paclitaxel?
Problem 55
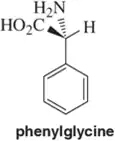
As we learned in Chapter 2, we don't need to show hydrogens bonded to carbons when drawing organic molecules using line-angle formulas. At asymmetric centers, however, we often show the hydrogen. Why? When might it be unnecessary to show the hydrogen at a chiral center?
Problem 57
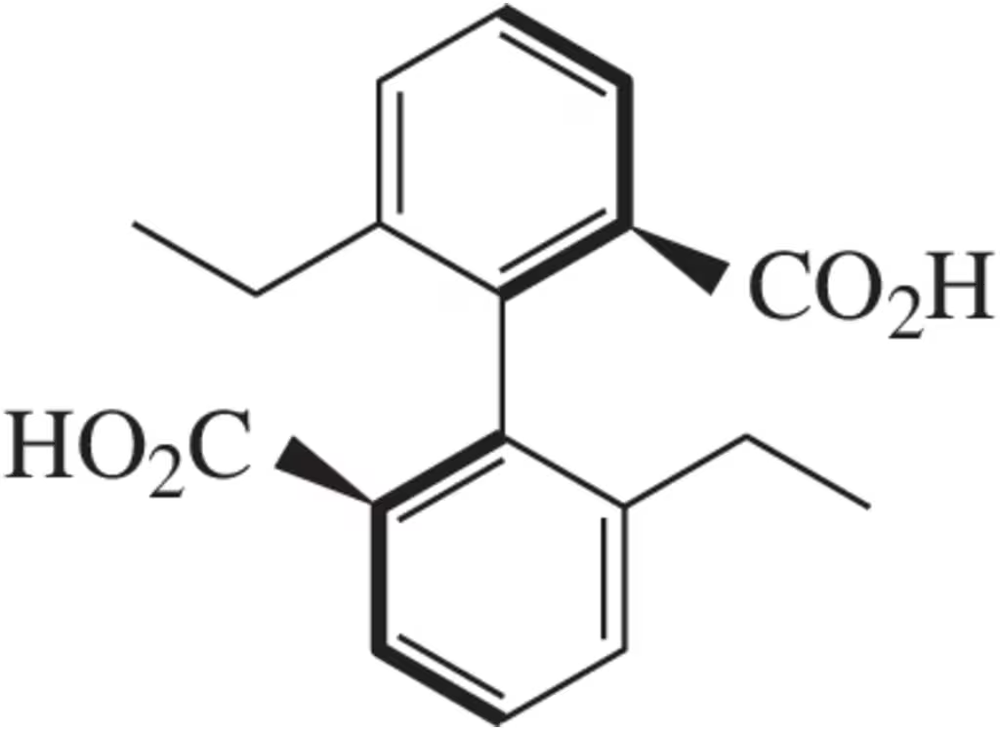
When the following substituted biphenyl was synthesized, it was found to have a specific rotation [α] of -23° at 25°C . When the specific rotation was measured at 100°C the compound had a specific rotation of 0° . Upon cooling back to 25°C the specific rotation was measured again, resulting in [α] = 0°. Explain these results.
Problem 58
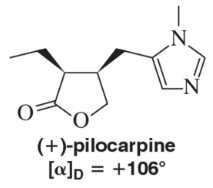
A chemist working on the synthesis of (+)-pilocarpine, an alkaloid used in the treatment of dry mouth and glaucoma, produced a mixture of enantiomers that gave a specific rotation [α]D = +97°. Based on the specific rotation of the pure enantiomer, calculate the ratio of (+)- to (-)-pilocarpine produced by the chemist.
Problem 59
A compound with two chiral centers that is meso will always have opposite absolute configurations at the two chiral centers. That is, a meso compound will never be (R,R) or (S,S); instead, it will be (R,S). Explain why this must be true.
Problem 60a
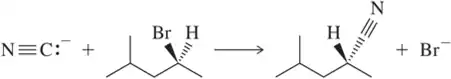
In Chapter 12, we introduce the SN2 reaction, a nucleophilic substitution reaction that proceeds with inversion. Confirm that inversion has occurred in each of the following examples by determining the absolute configuration of the chiral center in the reactants and products.
(a)
Problem 62
In Chapter 13, we explain how to convert secondary alcohols into ketones using a mild oxidation reaction. When the following enantiomerically pure and optically active secondary alcohol is submitted to these reaction conditions, the product is optically inactive. Explain this observation.
Problem 63
A chemist prepared a racemic mixture of the enantiomeric sulfonic acids shown here. Suggest two ways that these might be separated.
Problem 64
In contrast to Assessment 6.63, the following compounds should be easily separable using standard methods. Why?
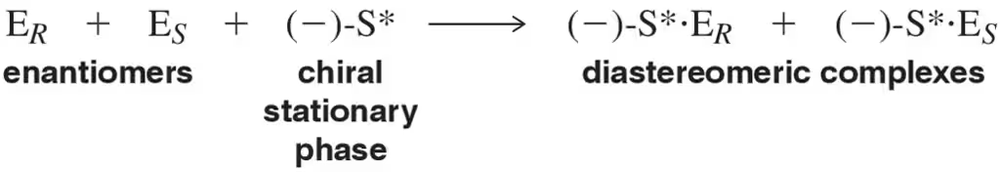
Problem 65
Chromatography using a chiral stationary phase is able to separate a pair of enantiomers because one of the enantiomers forms a more tightly bound complex with the stationary phase. Assuming that the (S) enantiomer elutes more slowly, rationalize this result on a reaction coordinate diagram.