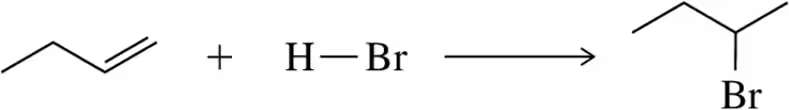Back
BackProblem 1b(ii,iii)
(ii) Which side of the reaction is favored by entropy? (iii) If ∆S° = 0 for these reactions, calculate ∆G° (Assume T = 298 K) [BDE for O―H = 110 kcal /mol.]
(b)
Problem 2a
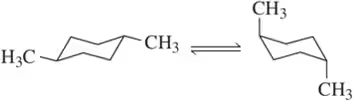
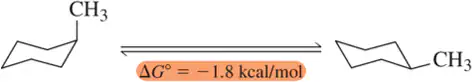
Predict which side of the equilibrium is favored for each of the chair–chair interconversions (ring flips) shown.
(a)
Problem 2c
Predict which side of the equilibrium is favored for each of the chair–chair interconversions (ring flips) shown.
(c)
Problem 3a
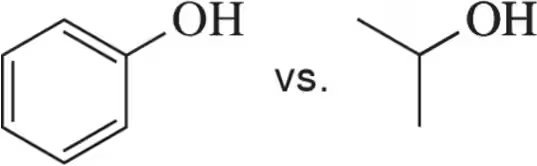
Which is the most stable base in each pair?
(a)
Problem 3b
Which is the most stable base in each pair?
(b) HS– and HO–
Problem 3c
Which is the most stable base in each pair?
(c) NH3 vs. H2O
Problem 4a
Which is the most acidic compound in each pair?
(a)
Problem 4b
Which is the most acidic compound in each pair?
(b) HF vs. HCl
Problem 4c
Which is the most acidic compound in each pair?
(c)
Problem 6a
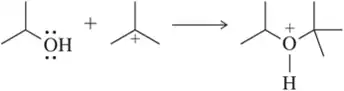
Provide a reasonable arrow-pushing mechanism for the following Lewis acid–Lewis base reactions.
(a)
Problem 6b
Provide a reasonable arrow-pushing mechanism for the following Lewis acid–Lewis base reactions.
(b)
Problem 7a
For the following equilibrium processes and the corresponding ∆G°, indicate whether you expect the equilibrium constant to be greater than, equal to, or less than 1. Justify your expectation in words.
(a)
Problem 7b
For the following equilibrium processes and the corresponding ∆G° , indicate whether you expect the equilibrium constant to be greater than, equal to, or less than 1. Justify your expectation in words.
(b)
Problem 7.2
Chapter 5 taught us that chemical reactions are random collisions. Experimentally, how can we make molecules collide more often?
Problem 10a
For the following equilibrium processes and the corresponding ∆G°, calculate (i) Keq and (ii) the % composition of the equilibrium mixture (% reactants, % products) at 298 K.
(a)
Problem 10b
For the following equilibrium processes and the corresponding ∆G°, calculate (i) Keq and (ii) the % composition of the equilibrium mixture (% reactants, % products) at 298 K.
(b)
Problem 11a
For the following values of ∆H° , ∆S°, and T, tell whether the process would be favored.
(a) ∆H° = -15 kcal/mol ; ∆S° = +37 cal/mol•K ; T = 273 K
Problem 11b
For the following values of ∆H° , ∆S°, and T, tell whether the process would be favored.
(b) ∆H° = +7.34 kcal/mol ; ∆S° = +43 cal/mol•K ; T = 325 K
Problem 11c
For the following values of ∆H° , ∆S°, and T, tell whether the process would be favored.
(c) ∆H° = -21.3 kcal/mol ; AS° = -51 cal/mol•K ; T = 373 K
Problem 11d
For the following values of ∆H° , ∆S°, and T, tell whether the process would be favored.
(d) ∆H° = -8.3 kcal/mol ; ∆S° = -12 cal/mol•K ; T = 298 K
Problem 12a
At what temperature does the entropy change of a process not contribute to the favorability of a process?
- Which of the following carbocations would you expect to undergo rearrangement?
Problem 13
Problem 13a
A certain process has ∆H° = 11.7 kcal/mol and AS° = +33cal/mol•K . That is, this reaction has an unfavorable enthalpy but a favorable entropy term. At what temperature will the process be neither favored nor disfavored?
Problem 14a
Considering the process described in Assessment 5.13, will it be favored or disfavored at a temperature higher than the one you calculated? How about at a temperature below what you calculated?
Problem 15a
Calculate ∆H° for the following equilibrium processes.
(a)
Problem 15b
Calculate ∆H° for the following equilibrium processes.
(b)
Problem 15d
Calculate ∆H° for the following equilibrium processes.
(d)
Problem 16a
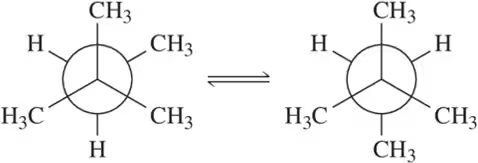
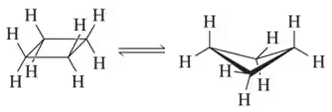
Which conformation in each of the following pairs has the least strain energy?
(a)
Problem 16b
Which conformation in each of the following pairs has the least strain energy?
(b)
Problem 16c
Which conformation in each of the following pairs has the least strain energy?
(c)