Back
BackProblem 17a
Identify the following substituted cycloalkanes as cis or trans.
(a)
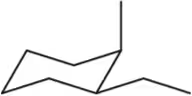
Problem 17c
Identify the following substituted cycloalkanes as cis or trans.
(c)
Problem 18a
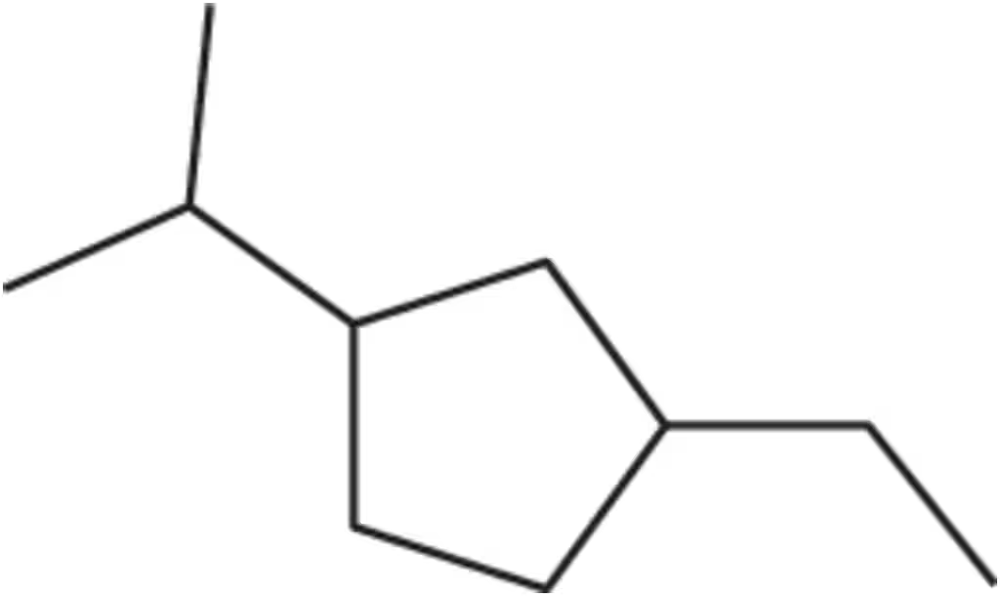
For the compound 1-ethyl-3-isopropylcyclopentane,
(a) draw two different cis and two different trans isomers.
Problem 18b
For the compound 1-ethyl-3-isopropylcyclopentane,
(b) What is the stereochemical relationship between the cis isomers?
Problem 18c
For the compound 1-ethyl-3-isopropylcyclopentane
(c) What is the stereochemical relationship between trans isomers?
Problem 18d
For the compound 1-ethyl-3-isopropylcyclopentane,
(d) LOOKING AHEAD What is the relationship between a cis isomer and a trans isomer?
Problem 19j
By comparing them to the models you created in Section 6.3.2, label the following chiral centers as R or S.
(j)
Problem 20a
Order the following sets of substituents via their priority using the CIP rules. (R = position of attachment to the asymmetric center.)
(a)
Problem 22a
Of the following pairs, identify the higher priority substituent according to the CIP rules. (R = position of attachment to the asymmetric center.)
(a)
Problem 24a
Prioritize the substituents at each chiral center and then, by comparing them to the models you created in Section 6.3.2, label the absolute configuration as R or S.
(a)
Problem 24d
Prioritize the substituents at each chiral center and then, by comparing them to the models you created in Section 6.3.2, label the absolute configuration as R or S.
(d)
Problem 24f
Prioritize the substituents at each chiral center and then, by comparing them to the models you created in Section 6.3.2, label the absolute configuration as R or S
(f)
Problem 25c
Prioritize the substituents at each chiral center and then, by each of the two methods discussed in Section 6.3.2.4, determine the absolute configuration. [Do not use your models, except to check your answers.]
(c)
Problem 25d
Prioritize the substituents at each chiral center and then, by each of the two methods discussed in Section 6.3.2.4, determine the absolute configuration. [Do not use your models, except to check your answers.]
(d)
Problem 25h
Prioritize the substituents at each chiral center and then, by each of the two methods discussed in Section 6.3.2.4, determine the absolute configuration. [Do not use your models, except to check your answers.]
(h)
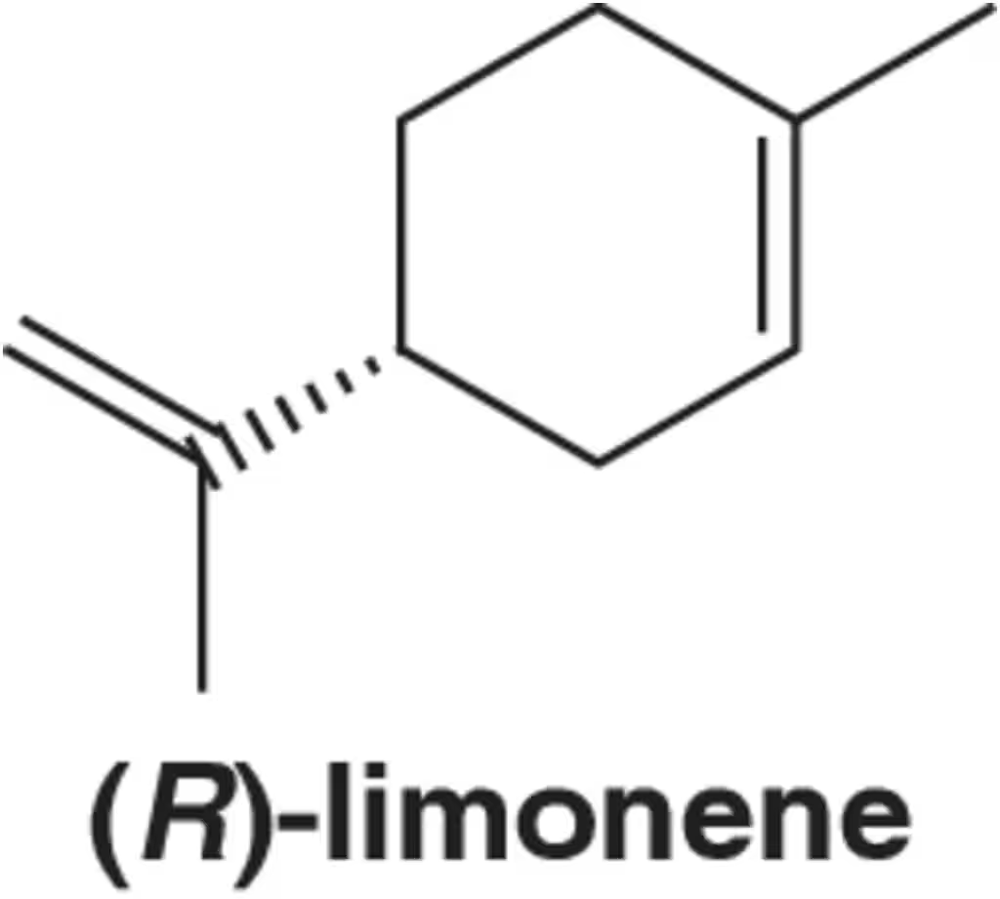
Problem 26
(R)-Limonene is a cyclic terpene responsible for the smell of oranges and other citrus fruits.
(a) Given that (R)-limonene rotates plane-polarized light in the clockwise direction, should it be referred to as (d) or (l)?
(b) Is it (+) or (-)?
(c) What direction (d or l; + or −) would you expect (S)-limonene to rotate plane-polarized light?
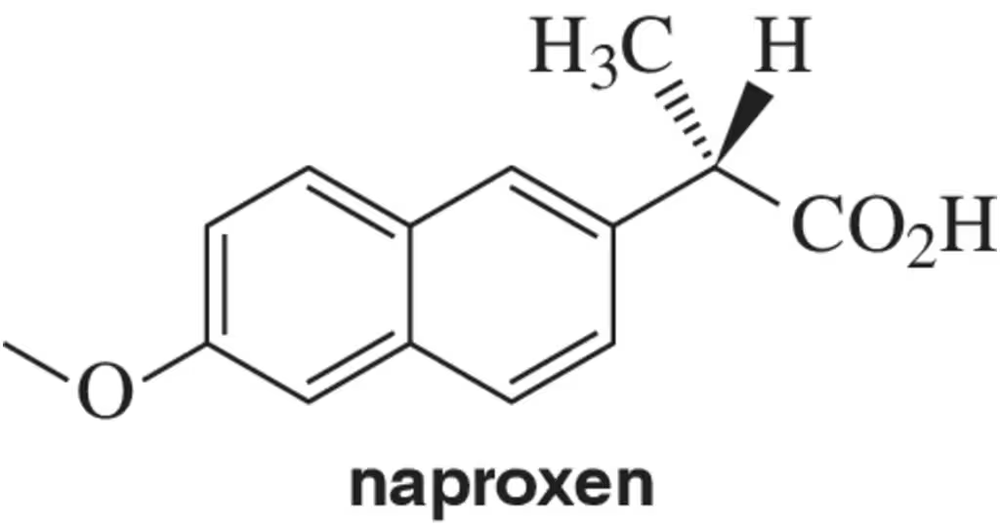
Problem 27a
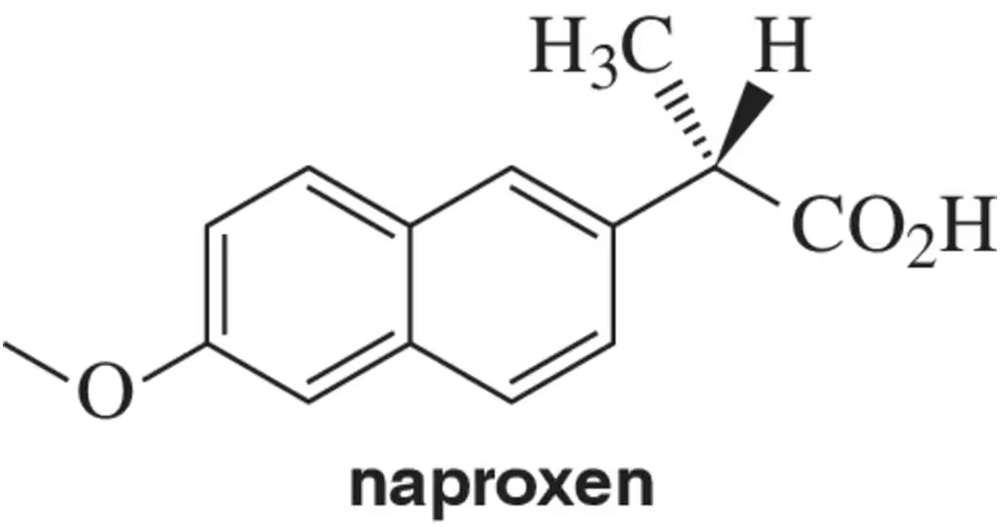
Naproxen is a commercially available anti-inflammatory sold under the name Aleve.
(a) Assign the absolute configuration as R or S.
Problem 27b,c,d
Naproxen is a commercially available anti-inflammatory sold under the name Aleve.
(b) Given that naproxen rotates plane-polarized light in the clockwise direction, should it be referred to as (d) or (l)?
(c) Is it (+) or (-)?
(d) What direction (d or l; + or −) would you expect the enantiomer of naproxen to rotate plane-polarized light?
Problem 28
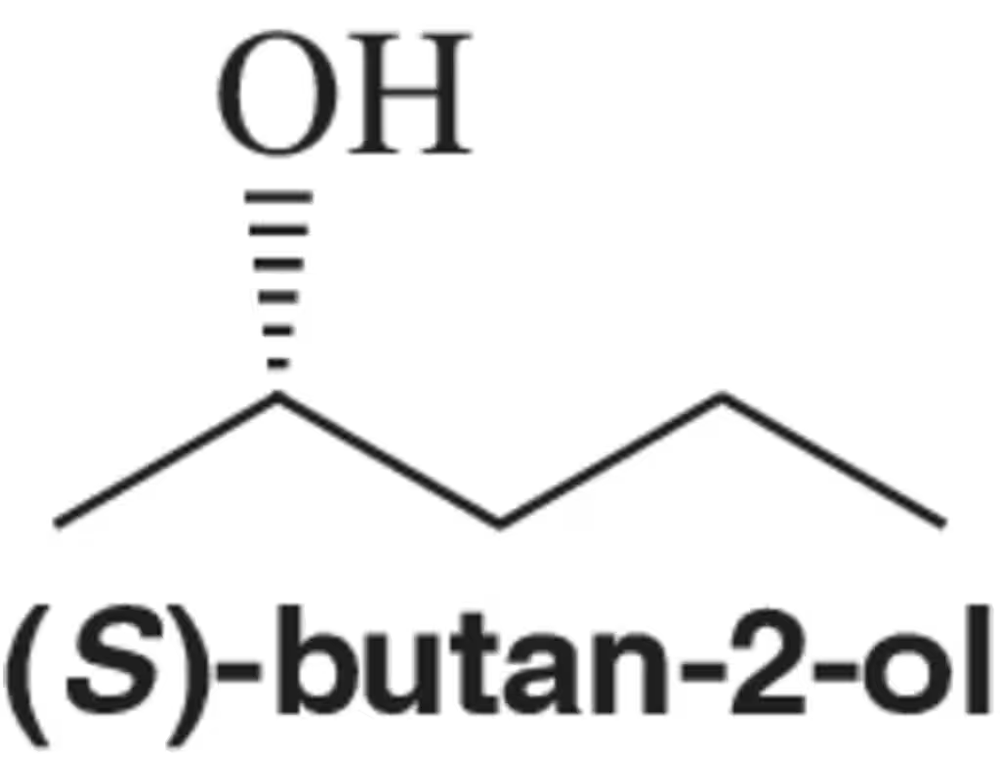
Based on the absolute configuration of (S)-butan-2-ol, what can you say about the direction it rotates plane-polarized light?
Problem 29
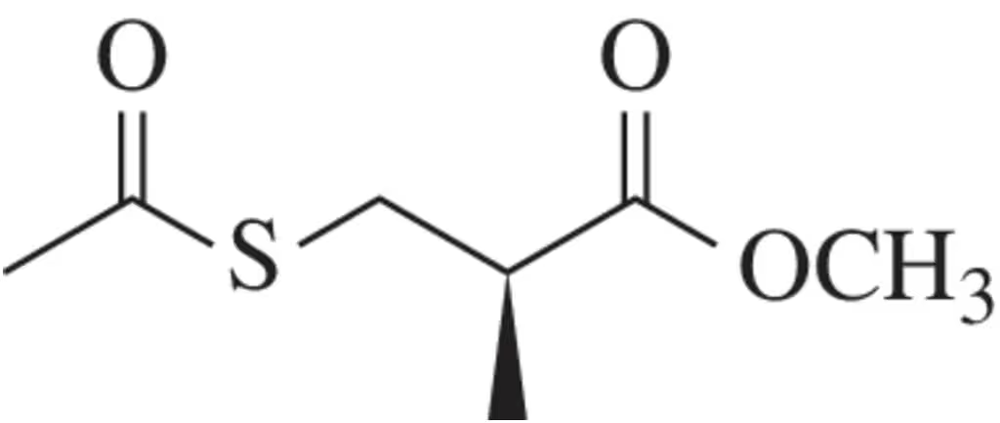
A student wanted to measure the specific rotation of the following propionate derivative (density = 1.12 g/ml).
A sample of the pure compound was placed in a 10.0-cm polarimeter tube, and using the sodium D line, the observed rotation at 20°C was determined to be +46.6° . What is the specific rotation of the propionate derivative?
Problem 30
Cholesterol (50 mg) was dissolved in 10 mL of chloroform and placed in a 1.0-cm polarimeter cell. This solution produced an observed rotation (using the sodium D line at 20°C ) of - 1.58° . What is the specific rotation of cholesterol?
Problem 31
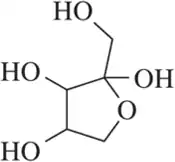
Using a 1.0-cm polarimeter cell and a solution prepared by dissolving 2.54 g of glucose in 1 L of H₂O, the specific rotation of d-glucose was calculated to be +52.7° at 20 °C using the sodium D line as the source of light. What was the observed rotation of the solution?
Problem 32
Imagine a sample that is enriched in the R enantiomer. If the % ee of the sample is 83%,
(a) what percent of the mixture is racemic?
(b) What is the ratio of R to S?
Problem 33
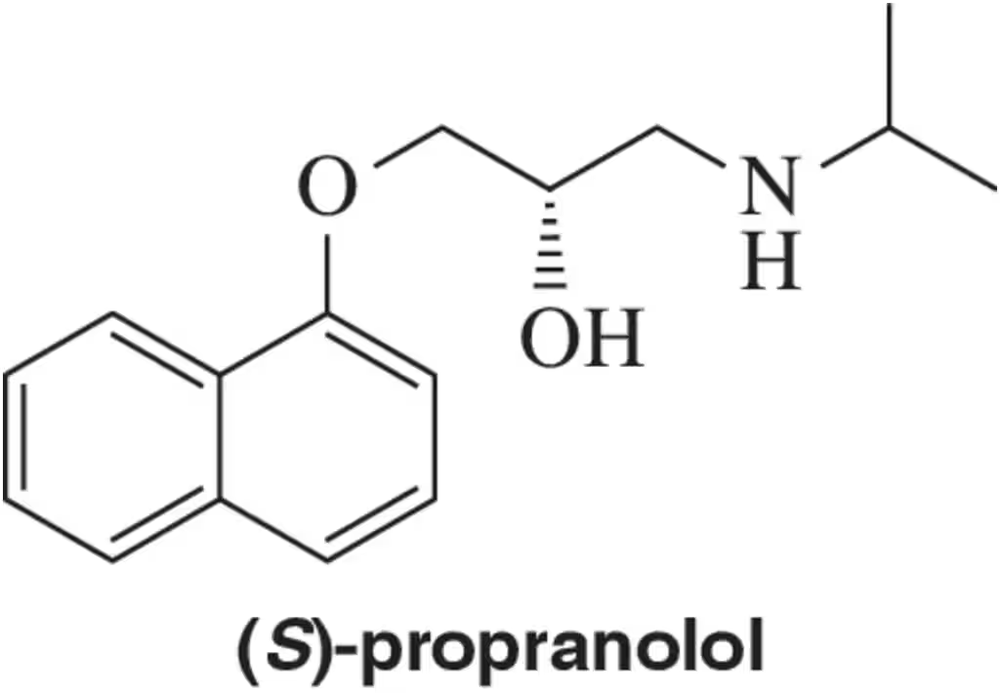
(S)-Propranolol, a drug used for the treatment of anxiety, has a specific rotation of -25.5° . Attempting to prepare it in enantiopure form, a chemist produced a compound that gave a specific rotation of -18.3° . What is the ratio of (S)- to (R)-propranolol produced by the chemist?
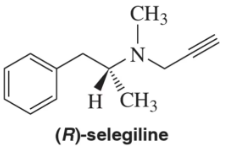
Problem 34
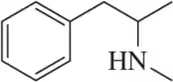
(R)-Selegiline, a monoamine oxidase (MAO) inactivator, was approved by the FDA in 1989 for the treatment of Parkinson's disease. In pure form, it has a specific rotation, [α]²⁰D = - 11.0°. What is the expected specific rotation of a mixture containing 64% S and 36% R?
Problem 35a
For the molecules shown,
(i) count the number of stereocenters present and
(ii) draw all possible stereoisomers.
(iii) Identify the relationships between stereoisomers as enantiomers or diastereomers.
(a)
Problem 35b
For the molecules shown,
(i) count the number of stereocenters present and
(ii) draw all possible stereoisomers.
(iii) Identify the relationships between stereoisomers as enantiomers or diastereomers.
(b)
Problem 35c
For the molecules shown,
(i) count the number of stereocenters present and
(ii) draw all possible stereoisomers.
(iii) Identify the relationships between stereoisomers as enantiomers or diastereomers.
(c)
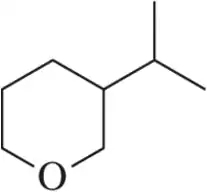
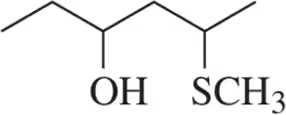
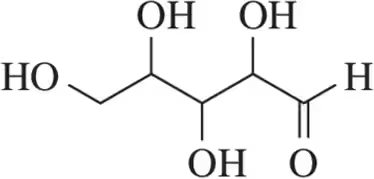
Problem 36a
How many stereoisomers are possible for each of the following molecules?
(a)
Problem 36c
How many stereoisomers are possible for each of the following molecules?
(c)