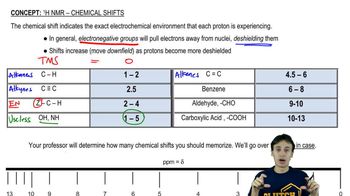
Draw the expected signal for a hydrogen with the following coupling constants.
(b) Hₐ : δ 3.34 (Jₐ꜀ = 9 , Jₐ₆ = 4 )

 Verified step by step guidance
Verified step by step guidance Verified video answer for a similar problem:
Verified video answer for a similar problem:

 11:3m
11:3mMaster Splitting with J-Values:Simple Tree Diagram with a bite sized video explanation from Johnny
Start learning