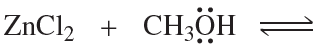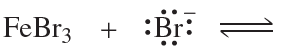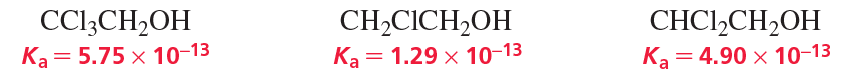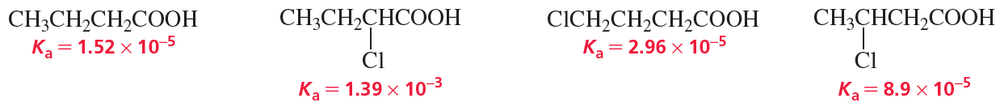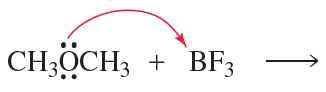Back
BackProblem 34c,d
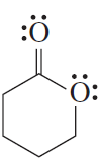
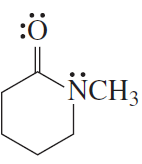
For each of the following compounds, indicate the atom that is protonated when an acid is added to a solution of the compound.
c.
d.
Problem 37
Fosamax (shown on the previous page) has six acidic groups. The active form of the drug, which has lost two of its acidic protons, is shown in the box.
(Notice that the phosphorus atom in Fosamax and the sulfur atom in [Problem 36] can be surrounded by more than eight electrons because P and S are below the second row of the periodic table.)
a. Why are the OH groups bonded to phosphorus the strongest acids of the six groups?
b. Which of the remaining four groups is the weakest acid?
Problem 38a,b,c
Using the table of pKa values given in [Appendix I], answer the following:
a. Which is the most acidic organic compound in the table?
b. Which is the least acidic organic compound in the table?
c. Which is the most acidic carboxylic acid in the table?
Problem 38d,e
Using the table of pKa values given in [Appendix I] , answer the following:
d. Which is more electronegative: an sp3 oxygen or an sp2 oxygen?
e. Which compounds demonstrate that the relative electronegativities of a hybridized nitrogen atom are sp > sp2 > sp3?
Problem 39a,b
For each of the following compounds (here shown in their acidic forms), write the form that predominates in a solution with a pH = 5.5:
a. CH3COOH(pKa = 4.76)
b. CH3CH2N+H3 (pKa = 11.0)
Problem 39c,d
For each of the following compounds (here shown in their acidic forms), write the form that predominates in a solution with a pH = 5.5:
c. H3O+ (pKa = −1.7)
d. HBr (pKa = −9)
Problem 39e,f
For each of the following compounds (here shown in their acidic forms), write the form that predominates in a solution with a pH = 5.5:
e. +NH4 (pKa = 9.4)
f. HC≡N (pKa = 9.1)
Problem 39g,h
For each of the following compounds (here shown in their acidic forms), write the form that predominates in a solution with a pH = 5.5:
g. HNO2 (pKa = 3.4)
h. HNO3 (pKa = −1.3)
Problem 39i
For each of the following compounds (here shown in their acidic forms), write the form that predominates in a solution with a pH = 5.5:
i. HON+H3 (pKa = 6.0)
Problem 40
As long as the pH is not less than ___________, at least 50% of a protonated amine with a pKa value of 10.4 will be in its neutral, nonprotonated form.
Problem 42a
Indicate whether a protonated amine (RN+H3) with a pKa value of 9 has more charged or more neutral molecules in a solution with the pH values given in Problem 41.
1. pH = 1
2. pH = 3
3. pH = 5
4. pH = 7
5. pH = 10
6. pH = 13
Problem 42b(1,2,3)
Indicate whether an alcohol (ROH) with a pKa value of 15 has more charged or more neutral molecules in a solution with the pH values given in Problem 41.
1. pH = 1
2. pH = 3
3. pH = 5
Problem 42b(3,4,5)
Indicate whether an alcohol (ROH) with a pKa value of 15 has more charged or more neutral molecules in a solution with the pH values given in Problem 41.
3. pH = 7
4. pH = 10
5. pH = 13
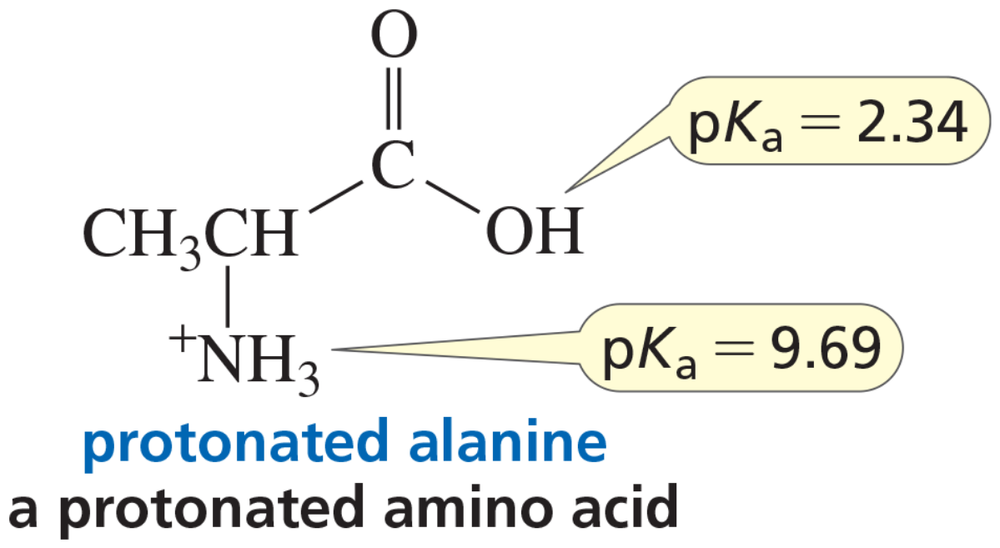
Problem 43c
A naturally occurring amino acid such as alanine has a group that is a carboxylic acid and a group that is a protonated amine. The pKa values of the two groups are shown.
c. Draw the structure of alanine in a solution at physiological pH (pH 7.4).
Problem 43d
A naturally occurring amino acid such as alanine has a group that is a carboxylic acid and a group that is a protonated amine. The pKa values of the two groups are shown.
d. Draw the structure of alanine in a solution at pH = 12.
Problem 43e,f
A naturally occurring amino acid such as alanine has a group that is a carboxylic acid and a group that is a protonated amine. The pKa values of the two groups are shown.
e. Is there a pH at which alanine is uncharged (that is, neither group has a charge)?
f. At what pH does alanine have no net charge (that is, the amount of negative charge is the same as the amount of positive charge)?
Problem 45a
At what pH is the concentration of a compound, with a pKa = 8.4, 100 times greater in its acidic form than in its basic form? At what pH is 50% of a compound, with a pKa = 7.3, in its basic form?
Problem 45c
At what pH is the concentration of a compound, with a pKa = 4.6, 10 times greater in its basic form than in its acidic form?
Problem 46a
For each of the following compounds, indicate the pH at which
a. 50% of the compound is in a form that possesses a charge.
1. CH3CH2COOH (pKa = 4.9)
2. CH3N+H3 (pKa = 10.7)
Problem 46b
For each of the following compounds, indicate the pH at which
b. more than 99% of the compound is in a form that possesses a charge.
1. CH3CH2COOH (pKa = 4.9)
2. CH3N+H3 (pKa = 10.7)
Problem 48a
Given the data in Problem 47:
a. What pH would you make the water layer to cause the carboxylic acid to dissolve in the water layer and the amine to dissolve in the ether layer?
Problem 48b
Given the data in Problem 47:
b. What pH would you make the water layer to cause the carboxylic acid to dissolve in the water layer and the amine to dissolve in the ether layer?
Problem 49
Write the equation that shows how a buffer made by dissolving CH3COOH and CH3COO−Na+ in water prevents the pH of a solution from changing appreciably when
a. a small amount of H+ is added to the solution.
b. a small amount of HO− is added to the solution.
Problem 51a,b
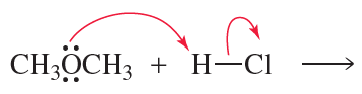
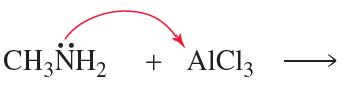
Draw the products of the following reactions. Use curved arrows to show where the pair of electrons starts and where it ends up.
a.
b.
Problem 52a,b
What products are formed when each of the following reacts with HO−?
a. CH3OH
b. +NH4
Problem 52c,d
What products are formed when each of the following reacts with HO− ?
c. CH3N+H3
d. BF3
Problem 52e,f
What products are formed when each of the following reacts with HO− ?
e. +CH3
f. FeBr3
Problem 55b
Explain the relative acidities.
Problem 56b,c
b. How does the presence of an electronegative substituent such as Cl affect the acidity of a carboxylic acid?
c. How does the location of the substituent affect the acidity of the carboxylic acid?
Problem 57
Draw the products of the following reactions:
a.
b.
c.