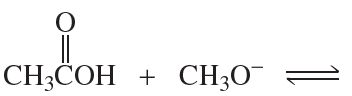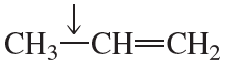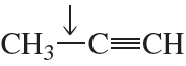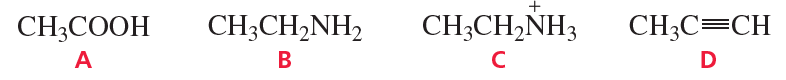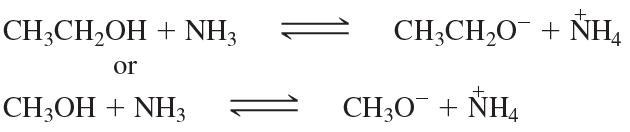Back
BackProblem 59
Rank the following compounds from strongest to weakest acid:
CH3CH2OH; CH3CH2NH2; CH3CH2SH; CH3CH2CH3
Problem 60a
For each of the following compounds, draw the form that predominates at pH = 3, pH = 6, pH = 10, and pH = 14:
a. CH3COOH (pKa = 4.8)
Problem 60b
For each of the following compounds, draw the form that predominates at pH = 3, pH = 6, pH = 10, and pH = 14:
b. CH3CH2N+H3 (pKa = 11.0)
Problem 60c
For each of the following compounds, draw the form that predominates at pH = 3, pH = 6, pH = 10, and pH = 14:
c. CF3CH2OH (pKa = 12.4)
Problem 61a
Give the products of the following acid–base reactions and indicate whether reactants or products are favored at equilibrium.
a.
Problem 61b
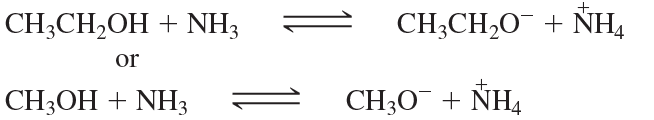
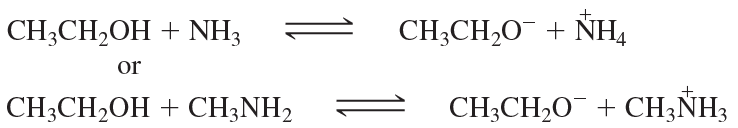
Give the products of the following acid–base reactions and indicate whether reactants or products are favored at equilibrium.
b. CH3CH2OH + -NH2 ⇌
Problem 62a
Rank the following alcohols from strongest to weakest acid.
CH2═CHCH2OH; CH3CH2CH2OH; HC≡CCH2OH
Problem 62b
Explain the relative acidities.
CH2═CHCH2OH, CH3CH2CH2OH, HC≡CCH2OH
Problem 63
A single bond between two carbons with different hybridizations has a small dipole. What is the direction of the dipole in the indicated bonds?
a.
b.
Problem 64
For each compound, indicate the atom that is most apt to be protonated.
a.
b.
c.
Problem 65a
Given the Ka values, estimate the pKa value of each of the following acids without using a calculator (that is, is it between 3 and 4, between 9 and 10, and so on?):
1. nitrous acid (HNO2), Ka = 4.0 × 10−4
2. nitric acid (HNO3), Ka = 22
3. bicarbonate (HCO3−), Ka = 6.3 × 10−11
4. hydrogen cyanide (HCN), Ka = 7.9 × 10−10
5. formic acid (HCOOH), Ka = 2.0 × 10−4
6. phosphoric acid (H3PO4), Ka = 2.1
Problem 65b,c
b. Determine the exact pKa values, using a calculator.
c. Which is the strongest acid?
1. nitrous acid (HNO2), Ka = 4.0 × 10−4
2. nitric acid (HNO3), Ka = 22
3. bicarbonate (HCO3−), Ka = 6.3 × 10−11
4. hydrogen cyanide (HCN), Ka = 7.9 × 10−10
5. formic acid (HCOOH), Ka = 2.0 × 10−4
6. phosphoric acid (H3PO4), Ka = 2.1
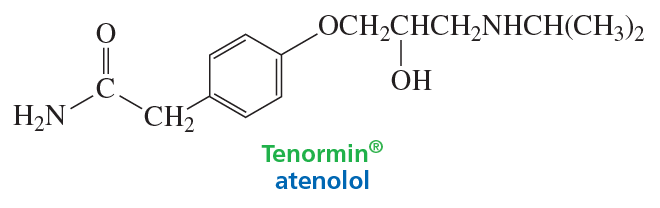
Problem 66
Tenormin, a member of the group of drugs known as beta-blockers, is used to treat high blood pressure and improve survival after a heart attack. It works by slowing down the heart to reduce its workload. Which atom in Tenormin is the most basic?
Problem 67
From which of the following compounds can HO− remove a proton in a reaction that favors product formation?
Problem 68a
For each of the following pairs of reactions, indicate which one has the more favorable equilibrium constant (that is, which one most favors products):
1.
2.
Problem 68b(1)
Which of the four reactions has the most favorable equilibrium constant?
1.
Problem 69
You are planning to carry out a reaction that produces protons. The reaction will be buffered at pH = 10.5. Would it be better to use a protonated methylamine/methylamine buffer or a protonated ethylamine/ethylamine buffer? (pKa of protonated methylamine = 10.7; pKa of protonated ethylamine = 11.0)
Problem 71
a. Without using a calculator, estimate the pH of each of the following solutions:
1. [HO−] = 3.2 × 10−5
2. [H3O+] = 8.3 × 10−1
3. [H3O+] = 1.7 × 10−3
b. Determine the exact pH, using a calculator.
Problem 72a
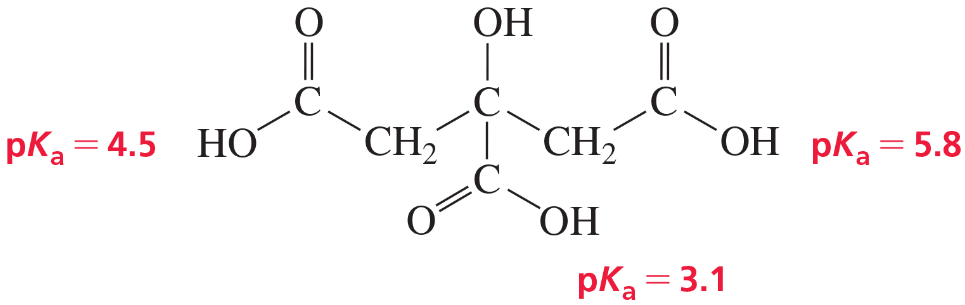
Citrus fruits are rich in citric acid, a compound with three COOH groups. Explain the following:
a. The first pKa (for the COOH group in the center of the molecule) is lower than the pKa of acetic acid.
Problem 72b
Citrus fruits are rich in citric acid, a compound with three COOH groups. Explain the following:
b. The third pKa is greater than the pKa of acetic acid.
Problem 73
Given that pH + pOH = 14 and that the concentration of water in a solution of water is 55.5 M, show that the pKa of water is 15.7. (Hint: pOH=−log [HO−])
Problem 74a
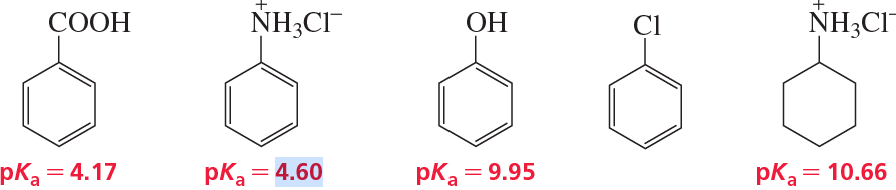
How could you separate a mixture of the following compounds? The reagents available to you are water, ether, 1.0 M HCl, and 1.0 M NaOH.
Problem 76a
If an acid with a pKa of 5.3 is in an aqueous solution of pH 5.7, what percentage of the acid is present in its acidic form?
Problem 76b
At what pH does 80% of the acid exist in its acidic form?