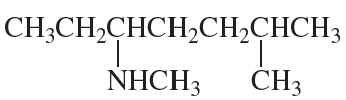Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.3 - An Introduction to Organic Compounds:Nomenclature, Physical Properties, and Structure
Ch.3 - An Introduction to Organic Compounds:Nomenclature, Physical Properties, and StructureProblem 71c,d
For rotation about the C-3---C-4 bond of 2-methylhexane, do the following:
c. About which other carbon–carbon bonds may rotation occur?
d. How many of the carbon–carbon bonds in the compound have staggered conformers that are all equally stable?
Problem 72a
Draw all the isomers that have molecular formula C5H11Br. (Hint: There are eight.)
a. Give the systematic name for each of the isomers.
Problem 72b
Draw all the isomers that have molecular formula C5H11Br. (Hint: There are eight.)
b. Give a common name for each isomer that has a common name.
Problem 72c,d,e
Draw all the isomers that have molecular formula C5H11Br. (Hint: There are eight.)
c. How many of the isomers are primary alkyl halides?
d. How many of the isomers are secondary alkyl halides?
e. How many of the isomers are tertiary alkyl halides?
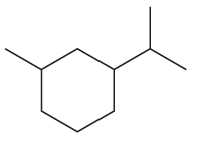
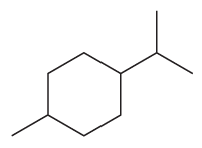
Problem 73g,h
What is each compound’s systematic name?
g.
h.
Problem 74b
Draw the two chair conformers for each of the following and indicate which conformer is more stable:
b. trans-1-ethyl-2-methylcyclohexane
Problem 74c
Draw the two chair conformers for each of the following and indicate which conformer is more stable:
c. trans-1-ethyl-2-isopropylcyclohexane
Problem 74d
Draw the two chair conformers for each of the following and indicate which conformer is more stable:
d. cis-1,2-diethylcyclohexane
Problem 74e
Draw the two chair conformers for each of the following and indicate which conformer is more stable:
e. cis-1-ethyl-3-isopropylcyclohexane
Problem 74f
Draw the two chair conformers for each of the following and indicate which conformer is more stable:
f. cis-1-ethyl-4-isopropylcyclohexane
Problem 75
Why are lower molecular weight alcohols more soluble in water than higher molecular weight alcohols?
Problem 76a
Draw a potential energy diagram for rotation about the C¬C bond of 1,2-dichloroethane through 360°, starting with the least stable conformer. The anti conformer is 1.2 kcal/mol more stable than a gauche conformer. A gauche conformer has two energy barriers, 5.2 kcal/mol and 9.3 kcal/mol.
Problem 76b
Draw the conformer that is present in greatest concentration.
Problem 76c
How much more stable is the most stable staggered conformer than the most stable eclipsed conformer?
Problem 76d
How much more stable is the most stable staggered conformer than the least stable eclipsed conformer?
Problem 77
For each of the following compounds, determine whether the cis isomer or the trans isomer is more stable.
a.
b.
c.
Problem 78
How many ethers have molecular formula C5H12O? Draw their structures and give each a systematic name. What are their common names?
Problem 79
Draw the most stable conformer of the following molecule. (A solid wedge points out of the plane of the paper toward the viewer. A hatched wedge points back from the plane of the paper away from the viewer.)
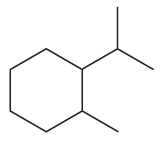
Problem 80a
What is each compound's systematic name?
a.
Problem 80b
What is each compound's systematic name?
b.
Problem 80c,d
What is each compound’s systematic name?
c.
d.
Problem 80e,f
What is each compound’s systematic name?
e.
f.
Problem 81
Calculate the energy difference between the two chair conformers of trans-1,2-dimethylcyclohexane.
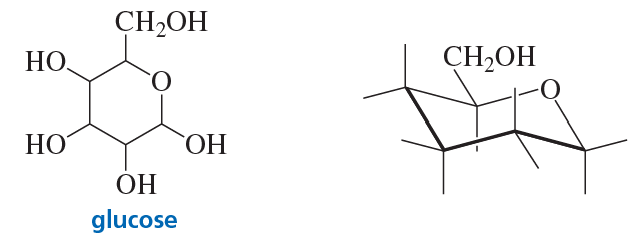
Problem 82
The most stable form of glucose (blood sugar) is a six-membered ring in a chair conformation with its five substituents all in equatorial positions. Draw the most stable conformer of glucose by putting the OH groups and hydrogens on the appropriate bonds in the structure on the right.
Problem 83e,f
What is each compound’s systematic name?
e.
f.
Problem 84a
Explain the following:
a. 1-Hexanol has a higher boiling point than 3-hexanol.
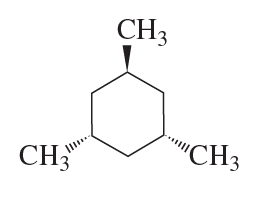
Problem 85
One of the chair conformers of cis-1,3-dimethylcyclohexane is 5.4 kcal/mol less stable than the other. How much steric strain does a 1,3-diaxial interaction between two methyl groups introduce into the conformer?
Problem 86
Bromine is a larger atom than chlorine, but the equilibrium constants in Table 3.9 indicate that a chloro substituent has a greater preference for the equatorial position than does a bromo substituent. Suggest an explanation for this fact.
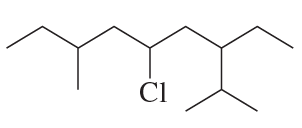
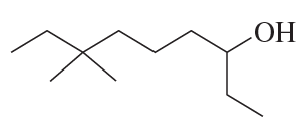
Problem 87b
Name the following compounds:
b.
Problem 87c
Name the following compounds:
c.