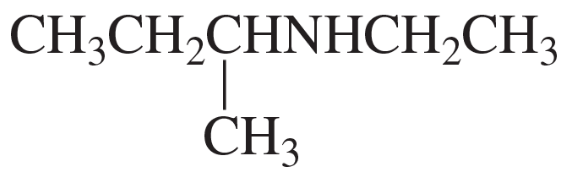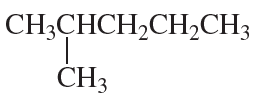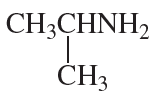Back
Back Bruice 8th Edition
Bruice 8th Edition Ch.3 - An Introduction to Organic Compounds:Nomenclature, Physical Properties, and Structure
Ch.3 - An Introduction to Organic Compounds:Nomenclature, Physical Properties, and StructureProblem 61
Draw a skeletal structure for an alkane that has
a. six carbons, all secondary.
b. eight carbons and only primary hydrogens.
c. seven carbons with two isopropyl groups.
Problem 62c
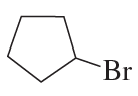
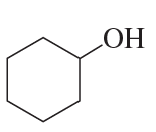
What is each compound's systematic name?
c.
Problem 62d
What is each compound's systematic name?
d. CH3CH2CH2OCH2CH3
Problem 62e
What is each compound's systematic name?
e.
Problem 62f
What is each compound's systematic name?
f.
Problem 62i,j
What is each compound's systematic name?
i.
j.
Problem 63a,b
Which has
a. the higher boiling point: 1-bromopentane or 1-bromohexane?
b. the higher boiling point: pentyl chloride or isopentyl chloride?
Problem 63c,d
Which has
c. the greater solubility in water: 1-butanol or 1-pentanol?
d. the higher boiling point: 1-hexanol or 1-methoxypentane?
Problem 63g
Which has
g. the higher boiling point: 1-bromopentane or 1-chloropentane?
Problem 63h
Which has
h. the higher boiling point: diethyl ether or butyl alcohol?
Problem 63i,j,k
Which has
i. the greater density: heptane or octane?
j. the higher boiling point: isopentyl alcohol or isopentylamine?
k. the higher boiling point: hexylamine or dipropylamine?
Problem 64c,d
c. Draw Newman projections of the two conformers of the trans isomer.
d. Which of the conformers predominates at equilibrium?
Problem 65
Ansaid and Motrin belong to the group of drugs known as nonsteroidal anti-inflammatory drugs (NSAIDs). Both are only slightly soluble in water, but one is a little more soluble than the other. Which of the drugs has the greater solubility in water?
Problem 66
Draw a picture of the hydrogen bonding in methanol.
Problem 67a,b
A student was given the structural formulas of several compounds and was asked to give them systematic names. How many did the student name correctly? Correct those that are misnamed.
a. 4-bromo-3-pentanol
b. 2,2-dimethyl-4-ethylheptane
Problem 67c,d
A student was given the structural formulas of several compounds and was asked to give them systematic names. How many did the student name correctly? Correct those that are misnamed.
c. 5-methylcyclohexanol
d. 1,1-dimethyl-2-cyclohexanol
Problem 67e,f
A student was given the structural formulas of several compounds and was asked to give them systematic names. How many did the student name correctly? Correct those that are misnamed.
e. 5-(2,2-dimethylethyl)nonane
f. isopentylbromide
Problem 67g
A student was given the structural formulas of several compounds and was asked to give them systematic names. How many did the student name correctly? Correct those that are misnamed.
g. 3,3-dichlorooctane
Problem 67h
A student was given the structural formulas of several compounds and was asked to give them systematic names. How many did the student name correctly? Correct those that are misnamed.
h. 5-ethyl-2-methylhexane
Problem 67k
A student was given the structural formulas of several compounds and was asked to give them systematic names. How many did the student name correctly? Correct those that are misnamed.
k. 2-methyl-2-isopropylheptane
Problem 67l
A student was given the structural formulas of several compounds and was asked to give them systematic names. How many did the student name correctly? Correct those that are misnamed.
l. 2-methyl-N,N-dimethyl-4-hexanamine
Problem 68
Which of the following conformers has the highest energy (is the least stable)?
Problem 69
Give the systematic names for all alkanes with molecular formula C7H16 that do not have any secondary hydrogens.
Problem 70a
Draw skeletal structures for the following:
a. 5-ethyl-2-methyloctane
Problem 70b
Draw skeletal structures for the following:
b. 1,3-dimethylcyclohexane
Problem 70c
Draw skeletal structures for the following:
c. 2,3,3,4-tetramethylheptane
Problem 70d
Draw skeletal structures for the following:
d. propylcyclopentane
Problem 70e
Draw skeletal structures for the following:
e. 2-methyl-4-(1-methylethyl)octane
Problem 70f
Draw skeletal structures for the following:
f. 2,6-dimethyl-4-(2-methylpropyl)decane
Problem 71a,b
For rotation about the C-3---C-4 bond of 2-methylhexane, do the following:
a. Draw the Newman projection of the most stable conformer.
b. Draw the Newman projection of the least stable conformer.