Back
BackProblem 69b,c,d
When 2-bromo-3-phenylbutane is treated with sodium methoxide, two alkenes result (by E2 elimination). The Zaitsev product predominates.
b. When one pure stereoisomer of 2-bromo-3-phenylbutane reacts, one pure stereoisomer of the major product results. For example, when (2R,3R)-2-bromo-3-phenylbutane reacts, the product is the stereoisomer with the methyl groups cis. Use your models to draw a Newman projection of the transition state to show why this stereospecificity is observed.
c. Use a Newman projection of the transition state to predict the major product of elimination of (2S,3R)-2-bromo-3-phenylbutane.
d. Predict the major product from elimination of (2S,3S)-2-bromo-3-phenylbutane. This prediction can be made without drawing any structures, by considering the results in part (b).
Problem 70
Explain the dramatic difference in rotational energy barriers of the following three alkenes. (Hint: Consider what the transition states must look like.)
Problem 71
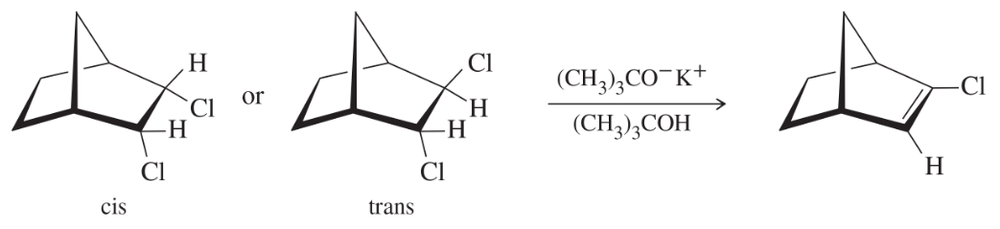
One of the following dichloronorbornanes undergoes elimination much faster than the other. Determine which one reacts faster, and explain the large difference in rates.
Problem 73
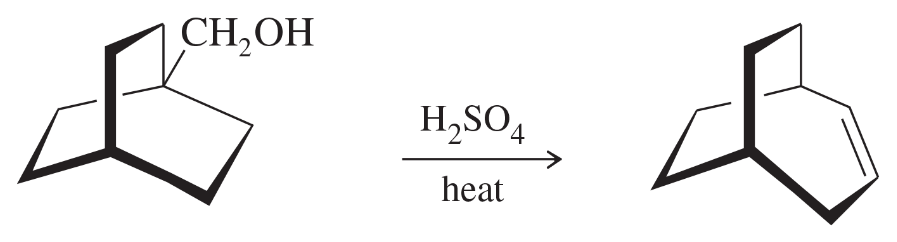
Write a mechanism that explains the formation of the following product. In your mechanism, explain the cause of the rearrangement, and explain the failure to form the Zaitsev product.
Problem 74
The following reaction is called the pinacol rearrangement. The reaction begins with an acid-promoted ionization to give a carbocation. This carbocation undergoes a methyl shift to give a more stable, resonance-stabilized cation. Loss of a proton gives the observed product. Propose a mechanism for the pinacol rearrangement.
Problem 75a
Deuterium (D) is the isotope of hydrogen of mass number 2, with a proton and a neutron in its nucleus. The chemistry of deuterium is nearly identical to the chemistry of hydrogen, except that the C―D bond is slightly (5.0 kJ/mol, or 1.2 kcal/mol) stronger than the C―H bond. Reaction rates tend to be slower if a C―D bond (as opposed to a C―H bond) is broken in a rate-limiting step. This effect on the rate is called a kinetic isotope effect. (Review PROBLEM 4-57)
a. Propose a mechanism to explain each product in the following reaction.
Problem 75b
Deuterium (D) is the isotope of hydrogen of mass number 2, with a proton and a neutron in its nucleus. The chemistry of deuterium is nearly identical to the chemistry of hydrogen, except that the C―D bond is slightly (5.0 kJ/mol, or 1.2 kcal/mol) stronger than the C―H bond. Reaction rates tend to be slower if a C―D bond (as opposed to a C―H bond) is broken in a rate-limiting step. This effect on the rate is called a kinetic isotope effect. (Review Problem 4-57)
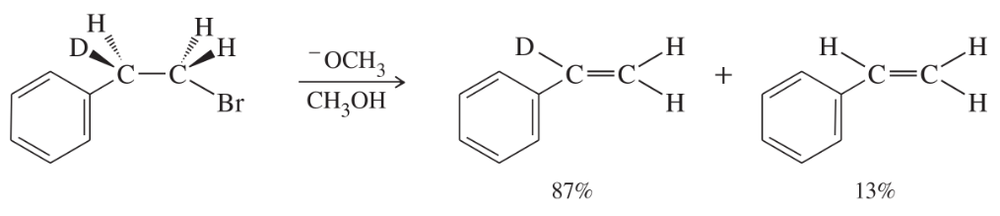
b. When the following deuterated compound reacts under the same conditions, the rate of formation of the substitution product is unchanged, while the rate of formation of the elimination product is slowed by a factor of 7.
Explain why the elimination rate is slower, but the substitution rate is unchanged.
Problem 76
When the following compound is treated with sodium methoxide in methanol, two elimination products are possible. Explain why the deuterated product predominates by about a 7:1 ratio (refer to Problem 7-75).